Oleic Acid Protects Endothelial Cells from Silica-Coated Superparamagnetic Iron Oxide Nanoparticles (SPIONs)-Induced Oxidative Stress and Cell Death
- PMID: 35806014
- PMCID: PMC9267005
- DOI: 10.3390/ijms23136972
Oleic Acid Protects Endothelial Cells from Silica-Coated Superparamagnetic Iron Oxide Nanoparticles (SPIONs)-Induced Oxidative Stress and Cell Death
Abstract
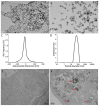
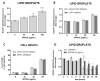
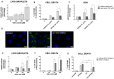
Superparamagnetic iron oxide nanoparticles (SPIONs) have great potential for use in medicine, but they may cause side effects due to oxidative stress. In our study, we investigated the effects of silica-coated SPIONs on endothelial cells and whether oleic acid (OA) can protect the cells from their harmful effects. We used viability assays, flow cytometry, infrared spectroscopy, fluorescence microscopy, and transmission electron microscopy. Our results show that silica-coated SPIONs are internalized by endothelial cells, where they increase the amount of reactive oxygen species (ROS) and cause cell death. Exposure to silica-coated SPIONs induced accumulation of lipid droplets (LD) that was not dependent on diacylglycerol acyltransferase (DGAT)-mediated LD biogenesis, suggesting that silica-coated SPIONs suppress LD degradation. Addition of exogenous OA promoted LD biogenesis and reduced SPION-dependent increases in oxidative stress and cell death. However, exogenous OA protected cells from SPION-induced cell damage even in the presence of DGAT inhibitors, implying that LDs are not required for the protective effect of exogenous OA. The molecular phenotype of the cells determined by Fourier transform infrared spectroscopy confirmed the destructive effect of silica-coated SPIONs and the ameliorative role of OA in the case of oxidative stress. Thus, exogenous OA protects endothelial cells from SPION-induced oxidative stress and cell death independent of its incorporation into triglycerides.
Keywords: endothelial cells; lipid droplets; oleic acid; oxidative stress; superparamagnetic iron oxide nanoparticles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Hedayatnasab Z., Dabbagh A., Abnisa F., Wan Daud W.M.A. Polycaprolactone-Coated Superparamagnetic Iron Oxide Nanoparticles for in Vitro Magnetic Hyperthermia Therapy of Cancer. Eur. Polym. J. 2020;133:109789. doi: 10.1016/j.eurpolymj.2020.109789. - DOI
-
- Samrot A.V., Sahithya C.S., Selvarani J., Purayil S.K., Ponnaiah P. A Review on Synthesis, Characterization and Potential Biological Applications of Superparamagnetic Iron Oxide Nanoparticles. Curr. Res. Green Sustain. Chem. 2021;4:100042. doi: 10.1016/j.crgsc.2020.100042. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

