Hydroxylation of Progesterone and Its Derivatives by the Entomopathogenic Strain Isaria farinosa KCh KW1.1
- PMID: 35806021
- PMCID: PMC9266320
- DOI: 10.3390/ijms23137015
Hydroxylation of Progesterone and Its Derivatives by the Entomopathogenic Strain Isaria farinosa KCh KW1.1
Abstract
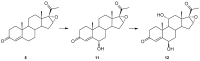
Progesterone biotransformation is worth studying because of the high industrial value of its derivatives. This study investigated the catalytic ability of the entomopathogenic filamentous fungus strain Isaria farinosa KCh KW1.1 to transform progesterone derivatives: 11α-hydroxyprogesterone, 17α-hydroxyprogesterone, 16α,17α-epoxyprogesterone and pregnenolone. In the culture of Isaria farinosa KCh KW1.1, 11α-hydroxyprogesterone was effectively transformed into only one product: 6β,11α-dihydroxyprogesterone. Transformation of 17α-hydroxyprogesterone gave three hydroxy derivatives: 6β,17α-dihydroxyprogesterone, 12β,17α-dihydroxyprogesterone and 6β,12β,17α-trihydroxyprogesterone. Two products: 6β-hydroxy-16α,17α-epoxyprogesterone and 6β,11α-dihydroxy-16α,17α-epoxyprogesterone, were obtained from the 16α,17α-epoxyprogesterone transformation. We isolated two compounds from the biotransformation medium with pregnenolone: 11α-hydroxy-7-oxopregnenolone and 5α,6α-epoxy-3β,11α-dihydroxypregnan-7,20-dione. In this study, we observed only mono- and dihydroxy derivatives of the tested substrates, and the number of obtained products for each biotransformation did not exceed three.
Keywords: Isaria farinosa; biotransformation; entomopathogenic fungi; progesterone derivatives.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Roglič U., Plazl I., Žnidaršič-Plazl P. Batch and Continuous Transformation of Progesterone by Rhizopus Nigricans Pellets in the Presence of β-Cyclodextrin. Biocatal. Biotransformation. 2007;25:16–23. doi: 10.1080/10242420601060954. - DOI
-
- Roglič U., Žnidaršič-Plazl P., Plazl I. The Influence of β-Cyclodextrin on the Kinetics of Progesterone Transformation by Rhizopus nigricans. Biocatal. Biotransformation. 2005;23:299–305. doi: 10.1080/10242420500175929. - DOI
-
- Žnidaršič P., Komel R., Pavko A. Studies of a Pelleted Growth Form of Rhizopus nigricans as a Biocatalyst for Progesterone 11α-Hydroxylation. J. Biotechnol. 1998;60:207–216. doi: 10.1016/S0168-1656(98)00010-8. - DOI
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources

