Regulation and Release of Vasoactive Endoglin by Brain Endothelium in Response to Hypoxia/Reoxygenation in Stroke
- PMID: 35806090
- PMCID: PMC9267030
- DOI: 10.3390/ijms23137085
Regulation and Release of Vasoactive Endoglin by Brain Endothelium in Response to Hypoxia/Reoxygenation in Stroke
Abstract
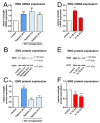
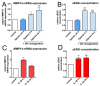
In large vessel occlusion stroke, recanalization to restore cerebral perfusion is essential but not necessarily sufficient for a favorable outcome. Paradoxically, in some patients, reperfusion carries the risk of increased tissue damage and cerebral hemorrhage. Experimental and clinical data suggest that endothelial cells, representing the interface for detrimental platelet and leukocyte responses, likely play a crucial role in the phenomenon referred to as ischemia/reperfusion (I/R)-injury, but the mechanisms are unknown. We aimed to determine the role of endoglin in cerebral I/R-injury; endoglin is a membrane-bound protein abundantly expressed by endothelial cells that has previously been shown to be involved in the maintenance of vascular homeostasis. We investigated the expression of membranous endoglin (using Western blotting and RT-PCR) and the generation of soluble endoglin (using an enzyme-linked immunosorbent assay of cell culture supernatants) after hypoxia and subsequent reoxygenation in human non-immortalized brain endothelial cells. To validate these in vitro data, we additionally examined endoglin expression in an intraluminal monofilament model of permanent and transient middle cerebral artery occlusion in mice. Subsequently, the effects of recombinant human soluble endoglin were assessed by label-free impedance-based measurement of endothelial monolayer integrity (using the xCELLigence DP system) and immunocytochemistry. Endoglin expression is highly inducible by hypoxia in human brain endothelial monolayers in vitro, and subsequent reoxygenation induced its shedding. These findings were corroborated in mice during MCAO; an upregulation of endoglin was displayed in the infarcted hemispheres under occlusion, whereas endoglin expression was significantly diminished after transient MCAO, which is indicative of shedding. Of note is the finding that soluble endoglin induced an inflammatory phenotype in endothelial monolayers. The treatment of HBMEC with endoglin resulted in a decrease in transendothelial resistance and the downregulation of VE-cadherin. Our data establish a novel mechanism in which hypoxia triggers the initial endothelial upregulation of endoglin and subsequent reoxygenation triggers its release as a vasoactive mediator that, when rinsed into adjacent vascular beds after recanalization, can contribute to cerebral reperfusion injury.
Keywords: CD105; HBMEC; endoglin; human brain endothelium; hypoxia; ischemia/reperfusion injury; middle cerebral artery occlusion; reoxygenation; soluble endoglin; stroke; vascular homeostasis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Mueller-Kronast N.H., Zaidat O.O., Froehler M.T., Jahan R., Aziz-Sultan M.A., Klucznik R.P., Saver J.L., Hellinger F.R., Jr., Yavagal D.R., Yao T.L., et al. Systematic Evaluation of Patients Treated With Neurothrombectomy Devices for Acute Ischemic Stroke: Primary Results of the STRATIS Registry. Stroke. 2017;48:2760–2768. doi: 10.1161/STROKEAHA.117.016456. - DOI - PubMed
-
- Knowland D., Arac A., Sekiguchi K.J., Hsu M., Lutz S.E., Perrino J., Steinberg G.K., Barres B.A., Nimmerjahn A., Agalliu D. Stepwise recruitment of transcellular and paracellular pathways underlies blood-brain barrier breakdown in stroke. Neuron. 2014;82:603–617. doi: 10.1016/j.neuron.2014.03.003. - DOI - PMC - PubMed
-
- Klohs J., Steinbrink J., Bourayou R., Mueller S., Cordell R., Licha K., Schirner M., Dirnagl U., Lindauer U., Wunder A. Near-infrared fluorescence imaging with fluorescently labeled albumin: A novel method for non-invasive optical imaging of blood-brain barrier impairment after focal cerebral ischemia in mice. J. Neurosci. Methods. 2009;180:126–132. doi: 10.1016/j.jneumeth.2009.03.002. - DOI - PubMed
-
- Rossi E., Pericacho M., Bachelot-Loza C., Pidard D., Gaussem P., Poirault-Chassac S., Blanco F.J., Langa C., Gonzalez-Manchon C., Novoa J.M.L., et al. Human endoglin as a potential new partner involved in platelet-endothelium interactions. Cell. Mol. Life Sci. 2018;75:1269–1284. doi: 10.1007/s00018-017-2694-7. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

