Cisplatin-Resistant CD44+ Lung Cancer Cells Are Sensitive to Auger Electrons
- PMID: 35806135
- PMCID: PMC9266901
- DOI: 10.3390/ijms23137131
Cisplatin-Resistant CD44+ Lung Cancer Cells Are Sensitive to Auger Electrons
Abstract
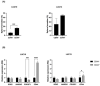
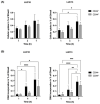
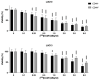
Cancer stem cells (CSCs) are resistant to conventional therapy and present a major clinical challenge since they are responsible for the relapse of many cancers, including non-small cell lung cancer (NSCLC). Hence, future successful therapy should also eradicate CSCs. Auger electrons have demonstrated promising therapeutic potential and can induce DNA damage while sparing surrounding cells. Here, we sort primary patient-derived NSCLC cells based on their expression of the CSC-marker CD44 and investigate the effects of cisplatin and a thymidine analog (deoxyuridine) labeled with an Auger electron emitter (125I). We show that the CD44+ populations are more resistant to cisplatin than the CD44- populations. Interestingly, incubation with the thymidine analog 5-[125I]iodo-2'-deoxyuridine ([125I]I-UdR) induces equal DNA damage, G2/M cell cycle arrest, and apoptosis in the CD44- and CD44+ populations. Our results suggest that Auger electron emitters can also eradicate resistant lung cancer CD44+ populations.
Keywords: CD44; DNA damage; apoptosis; auger electrons; cancer stem cells; cisplatin; lung cancer.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
Figures






References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

