New 2-[(4-Amino-6- N-substituted-1,3,5-triazin-2-yl)methylthio]- N-(imidazolidin-2-ylidene)-4-chloro-5-methylbenzenesulfonamide Derivatives, Design, Synthesis and Anticancer Evaluation
- PMID: 35806186
- PMCID: PMC9267128
- DOI: 10.3390/ijms23137178
New 2-[(4-Amino-6- N-substituted-1,3,5-triazin-2-yl)methylthio]- N-(imidazolidin-2-ylidene)-4-chloro-5-methylbenzenesulfonamide Derivatives, Design, Synthesis and Anticancer Evaluation
Abstract
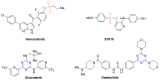
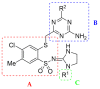
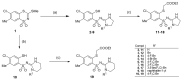
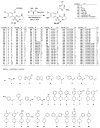
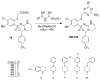
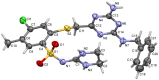
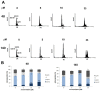
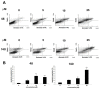
In the search for new compounds with antitumor activity, new potential anticancer agents were designed as molecular hybrids containing the structures of a triazine ring and a sulfonamide fragment. Applying the synthesis in solution, a base of new sulfonamide derivatives 20-162 was obtained by the reaction of the corresponding esters 11-19 with appropriate biguanide hydrochlorides. The structures of the compounds were confirmed by spectroscopy (IR, NMR), mass spectrometry (HRMS or MALDI-TOF/TOF), elemental analysis (C,H,N) and X-ray crystallography. The cytotoxic activity of the obtained compounds toward three tumor cell lines, HCT-116, MCF-7 and HeLa, was examined. The results showed that some of the most active compounds belonged to the R1 = 4-trifluoromethylbenzyl and R1 = 3,5-bis(trifluoromethyl)benzyl series and exhibited IC50 values ranging from 3.6 µM to 11.0 µM. The SAR relationships were described, indicating the key role of the R2 = 4-phenylpiperazin-1-yl substituent for the cytotoxic activity against the HCT-116 and MCF-7 lines. The studies regarding the mechanism of action of the active compounds included the assessment of the inhibition of MDM2-p53 interactions, cell cycle analysis and apoptosis induction examination. The results indicated that the studied compounds did not inhibit MDM2-p53 interactions but induced G0/G1 and G2/M cell cycle arrest in a p53-independent manner. Furthermore, the active compounds induced apoptosis in cells harboring wild-type and mutant p53. The compound design was conducted step by step and assisted by QSAR models that correlated the activity of the compounds against the HCT-116 cell line with molecular descriptors.
Keywords: 1,3,5-triazines; QSAR; anticancer activity; apoptosis; benzenesulfonamide; cell cycle arrest; imidazole; proliferation; synthesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
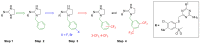





References
-
- World Health Organization (WHO) Fact Sheet: Cancer. [(accessed on 3 February 2022)]. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer.
-
- Hong X., Li Z.-X., Hou J., Zhang H.-Y., Zhang C.-Y., Zhang J., Sun H., Pang L.-H., Wang T., Deng Z.-H. Effects of ER-resident and secreted AGR2 on cell proliferation, migration, invasion, and survival in PANC-1 pancreatic cancer cells. BMC Cancer. 2021;21:33. doi: 10.1186/s12885-020-07743-y. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

