Glioblastoma Extracellular Vesicle-Specific Peptides Inhibit EV-Induced Neuronal Cytotoxicity
- PMID: 35806205
- PMCID: PMC9266738
- DOI: 10.3390/ijms23137200
Glioblastoma Extracellular Vesicle-Specific Peptides Inhibit EV-Induced Neuronal Cytotoxicity
Abstract
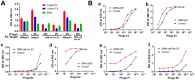
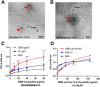
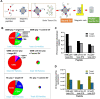
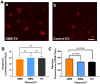
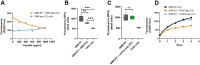
WHO Grade 4 IDH-wild type astrocytoma (GBM) is the deadliest brain tumor with a poor prognosis. Meningioma (MMA) is a more common "benign" central nervous system tumor but with significant recurrence rates. There is an urgent need for brain tumor biomarkers for early diagnosis and effective treatment options. Extracellular vesicles (EVs) are tiny membrane-enclosed vesicles that play essential functions in cell-to-cell communications among tumor cells. We aimed to identify epitopes of brain tumor EVs by phage peptide libraries. EVs from GBM plasma, MMA plasma, or brain tumor cell lines were used to screen phage-displayed random peptide libraries to identify high-affinity peptides. We purified EVs from three GBM plasma pools (23 patients), one MMA pool (10 patients), and four brain tumor cell lines. We identified a total of 21 high-affinity phage peptides (12 unique) specific to brain tumor EVs. The peptides shared high sequence homologies among those selected by the same EVs. Dose-response ELISA demonstrated that phage peptides were specific to brain tumor EVs compared to controls. Peptide affinity purification identified unique brain tumor EV subpopulations. Significantly, GBM EV peptides inhibit brain tumor EV-induced complement-dependent cytotoxicity (necrosis) in neurons. We conclude that phage display technology could identify specific peptides to isolate and characterize tumor EVs.
Keywords: ELISA; cytotoxicity; extracellular vesicles; glioblastoma; meningioma; neurons; peptides; phage-display; plasma; tumor cell line.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Louis D.N., Perry A., Wesseling P., Brat D.J., Cree I.A., Figarella-Branger D., Hawkins C., Ng H.K., Pfister S.M., Reifenberger G., et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncology. 2021;23:1231–1251. doi: 10.1093/neuonc/noab106. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

