Iron, Neuroinflammation and Neurodegeneration
- PMID: 35806270
- PMCID: PMC9266893
- DOI: 10.3390/ijms23137267
Iron, Neuroinflammation and Neurodegeneration
Abstract
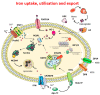
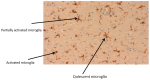
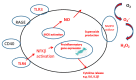
Disturbance of the brain homeostasis, either directly via the formation of abnormal proteins or cerebral hypo-perfusion, or indirectly via peripheral inflammation, will activate microglia to synthesise a variety of pro-inflammatory agents which may lead to inflammation and cell death. The pro-inflammatory cytokines will induce changes in the iron proteins responsible for maintaining iron homeostasis, such that increased amounts of iron will be deposited in cells in the brain. The generation of reactive oxygen and nitrogen species, which is directly involved in the inflammatory process, can significantly affect iron metabolism via their interaction with iron-regulatory proteins (IRPs). This underlies the importance of ensuring that iron is maintained in a form that can be kept under control; hence, the elegant mechanisms which have become increasingly well understood for regulating iron homeostasis. Therapeutic approaches to minimise the toxicity of iron include N-acetyl cysteine, non-steroidal anti-inflammatory compounds and iron chelation.
Keywords: astrocytes; iron; iron homeostasis; microglia; neuroinflammation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Crichton R.R. Iron Metabolism. From Molecular Mechanisms to Cellular Consequences. 4th ed. John Wiley and Sons; Chichester, UK: 2016. p. 556.
-
- Santana-Codina N., Gikandi A., Mancias J.D. The Role of NCOA4-Mediated Ferritinophagy in Ferroptosis. Adv. Exp. Med. Biol. 2021;1301:41–57. - PubMed
-
- Yanatori I., Richardson D.R., Toyokuni S., Kishi F. The new role of poly (rC)-binding proteins as iron transport chaperones: Proteins that could couple with inter-organelle interactions to safely traffic iron. Biochim. Biophys. Acta Gen. Subj. 2020;1864:129685. doi: 10.1016/j.bbagen.2020.129685. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

