Adipose Tissue Inflammation and Pulmonary Dysfunction in Obesity
- PMID: 35806353
- PMCID: PMC9267094
- DOI: 10.3390/ijms23137349
Adipose Tissue Inflammation and Pulmonary Dysfunction in Obesity
Abstract
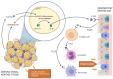
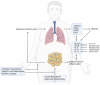
Obesity is a chronic disease caused by an excess of adipose tissue that may impair health by altering the functionality of various organs, including the lungs. Excessive deposition of fat in the abdominal area can lead to abnormal positioning of the diaphragm and consequent reduction in lung volume, leading to a heightened demand for ventilation and increased exposure to respiratory diseases, such as chronic obstructive pulmonary disease, asthma, and obstructive sleep apnoea. In addition to mechanical ventilatory constraints, excess fat and ectopic deposition in visceral depots can lead to adipose tissue dysfunction, which promotes metabolic disorders. An altered adipokine-secretion profile from dysfunctional adipose tissue in morbid obesity fosters systemic, low-grade inflammation, impairing pulmonary immune response and promoting airway hyperresponsiveness. A potential target of these adipokines could be the NLRP3 inflammasome, a critical component of the innate immune system, the harmful pro-inflammatory effect of which affects both adipose and lung tissue in obesity. In this review, we will investigate the crosstalk between adipose tissue and the lung in obesity, highlighting the main inflammatory mediators and novel therapeutic targets in preventing pulmonary dysfunction.
Keywords: adipokines; adipose tissue; crosstalk; inflammasome; lung; obesity; respiratory diseases.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Cignarelli A., Ciavarella A., Barbaro M., Kounaki S., Di Trani A., Falcone V.A., Quaranta V.N., Natalicchio A., Laviola L., Resta O., et al. Postprandial glucose and HbA1c are associated with severity of obstructive sleep apnoea in non-diabetic obese subjects. J. Endocrinol. Investig. 2021;44:2741–2748. doi: 10.1007/s40618-021-01602-8. - DOI - PMC - PubMed
-
- Perrini S., Cignarelli A., Quaranta V.N., Falcone V.A., Kounaki S., Porro S., Ciavarella A., Ficarella R., Barbaro M., Genchi V.A., et al. Correction of intermittent hypoxia reduces inflammation in obese subjects with obstructive sleep apnea. JCI Insight. 2017;2:e94379. doi: 10.1172/jci.insight.94379. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

