Ca2+ Signalling and Hypoxia/Acidic Tumour Microenvironment Interplay in Tumour Progression
- PMID: 35806388
- PMCID: PMC9266881
- DOI: 10.3390/ijms23137377
Ca2+ Signalling and Hypoxia/Acidic Tumour Microenvironment Interplay in Tumour Progression
Abstract
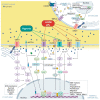
Solid tumours are characterised by an altered microenvironment (TME) from the physicochemical point of view, displaying a highly hypoxic and acidic interstitial fluid. Hypoxia results from uncontrolled proliferation, aberrant vascularization and altered cancer cell metabolism. Tumour cellular apparatus adapts to hypoxia by altering its metabolism and behaviour, increasing its migratory and metastatic abilities by the acquisition of a mesenchymal phenotype and selection of aggressive tumour cell clones. Extracellular acidosis is considered a cancer hallmark, acting as a driver of cancer aggressiveness by promoting tumour metastasis and chemoresistance via the selection of more aggressive cell phenotypes, although the underlying mechanism is still not clear. In this context, Ca2+ channels represent good target candidates due to their ability to integrate signals from the TME. Ca2+ channels are pH and hypoxia sensors and alterations in Ca2+ homeostasis in cancer progression and vascularization have been extensively reported. In the present review, we present an up-to-date and critical view on Ca2+ permeable ion channels, with a major focus on TRPs, SOCs and PIEZO channels, which are modulated by tumour hypoxia and acidosis, as well as the consequent role of the altered Ca2+ signals on cancer progression hallmarks. We believe that a deeper comprehension of the Ca2+ signalling and acidic pH/hypoxia interplay will break new ground for the discovery of alternative and attractive therapeutic targets.
Keywords: Ca2+ signalling; PIEZO channels; SOC channels; TRP channels; hypoxia; tumour acidic microenvironment; tumour progression.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Ždralević M., Brand A., Di Ianni L., Dettmer K., Reinders J., Singer K., Peter K., Schnell A., Bruss C., Decking S.-M., et al. Double Genetic Disruption of Lactate Dehydrogenases A and B Is Required to Ablate the “Warburg Effect” Restricting Tumour Growth to Oxidative Metabolism. J. Biol. Chem. 2018;293:15947–15961. doi: 10.1074/jbc.RA118.004180. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

