Do Extracellular Vesicles Derived from Mesenchymal Stem Cells Contain Functional Mitochondria?
- PMID: 35806411
- PMCID: PMC9266972
- DOI: 10.3390/ijms23137408
Do Extracellular Vesicles Derived from Mesenchymal Stem Cells Contain Functional Mitochondria?
Abstract
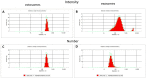
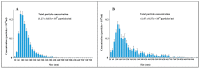
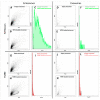
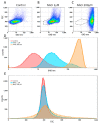
Extracellular vesicles (EV) derived from stem cells have become an effective complement to the use in cell therapy of stem cells themselves, which has led to an explosion of research into the mechanisms of vesicle formation and their action. There is evidence demonstrating the presence of mitochondrial components in EV, but a definitive conclusion about whether EV contains fully functional mitochondria has not yet been made. In this study, two EV fractions derived from mesenchymal stromal stem cells (MSC) and separated by their size were examined. Flow cytometry revealed the presence of mitochondrial lipid components capable of interacting with mitochondrial dyes MitoTracker Green and 10-nonylacridine orange; however, the EV response to the probe for mitochondrial membrane potential was negative. Detailed analysis revealed components from all mitochondria compartments, including house-keeping mitochondria proteins and DNA as well as energy-related proteins such as membrane-localized proteins of complexes I, IV, and V, and soluble proteins from the Krebs cycle. When assessing the functional activity of mitochondria, high variability in oxygen consumption was noted, which was only partially attributed to mitochondrial respiratory activity. Our findings demonstrate that the EV contain all parts of mitochondria; however, their independent functionality inside EV has not been confirmed, which may be due either to the absence of necessary cofactors and/or the EV formation process and, probably the methodology of obtaining EV.
Keywords: ectosomes; exosomes; extracellular vesicles; mesenchymal stromal cells; mitochondria; stem cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Yáñez-Mó M., Siljander P.R.-M., Andreu Z., Zavec A.B., Borràs F.E., Buzas E.I., Buzas K., Casal E., Cappello F., Carvalho J., et al. Biological Properties of Extracellular Vesicles and Their Physiological Functions. J. Extracell. Vesicles. 2015;4:27066. doi: 10.3402/jev.v4.27066. - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

