Fenretinide in Cancer and Neurological Disease: A Two-Face Janus Molecule
- PMID: 35806431
- PMCID: PMC9266536
- DOI: 10.3390/ijms23137426
Fenretinide in Cancer and Neurological Disease: A Two-Face Janus Molecule
Abstract
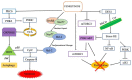
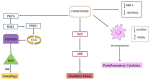
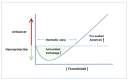
Recently, several chemotherapeutic drugs have been repositioned in neurological diseases, based on common biological backgrounds and the inverse comorbidity between cancer and neurodegenerative diseases. Fenretinide (all-trans-N-(4-hydroxyphenyl) retinamide, 4-HPR) is a synthetic derivative of all-trans-retinoic acid initially proposed in anticancer therapy for its antitumor effects combined with limited toxicity. Subsequently, fenretinide has been proposed for other diseases, for which it was not intentionally designed for, due to its ability to influence different biological pathways, providing a broad spectrum of pharmacological effects. Here, we review the most relevant preclinical and clinical findings from fenretinide and discuss its therapeutic role towards cancer and neurological diseases, highlighting the hormetic behavior of this pleiotropic molecule.
Keywords: anticancer drugs; fenretinide; hormesis; nanomicellar formulations; neuroinflammation; neuroprotection; oxidative stress; repositioning.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Greco A., Valle J.S., Pancaldi V., Baudot A., Barillot E., Caselle M., Valencia A., Zinovyev A., Cantini L. Molecular Inverse Comorbidity between Alzheimer’s Disease and Lung Cancer: New Insights from Matrix Factorization. Int. J. Mol. Sci. 2019;20:3114. doi: 10.3390/ijms20133114. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

