Lysophosphatidic Acid Is a Proinflammatory Stimulus of Renal Tubular Epithelial Cells
- PMID: 35806457
- PMCID: PMC9267536
- DOI: 10.3390/ijms23137452
Lysophosphatidic Acid Is a Proinflammatory Stimulus of Renal Tubular Epithelial Cells
Abstract
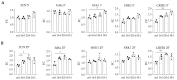
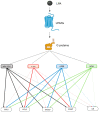
Chronic kidney disease (CKD) refers to a spectrum of diseases defined by renal fibrosis, permanent alterations in kidney structure, and low glomerular-filtration rate. Prolonged epithelial-tubular damage involves a series of changes that eventually lead to CKD, highlighting the importance of tubular epithelial cells in this process. Lysophosphatidic acid (LPA) is a bioactive lipid that signals mainly through its six cognate LPA receptors and is implicated in several chronic inflammatory pathological conditions. In this report, we have stimulated human proximal tubular epithelial cells (HKC-8) with LPA and 175 other possibly pathological stimuli, and simultaneously detected the levels of 27 intracellular phosphoproteins and 32 extracellular secreted molecules with multiplex ELISA. This quantification revealed a large amount of information concerning the signaling and the physiology of HKC-8 cells that can be extrapolated to other proximal tubular epithelial cells. LPA responses clustered with pro-inflammatory stimuli such as TNF and IL-1, promoting the phosphorylation of important inflammatory signaling hubs, including CREB1, ERK1, JUN, IκΒα, and MEK1, as well as the secretion of inflammatory factors of clinical relevance, including CCL2, CCL3, CXCL10, ICAM1, IL-6, and IL-8, most of them shown for the first time in proximal tubular epithelial cells. The identified LPA-induced signal-transduction pathways, which were pharmacologically validated, and the secretion of the inflammatory factors offer novel insights into the possible role of LPA in CKD pathogenesis.
Keywords: cytokines; inflammation; lysophosphatidic acid; tubular epithelial cells.
Conflict of interest statement
C.U. is an employee of F. Hoffmann-La Roche AG.
Figures



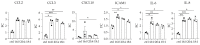



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

