A Novel Artificial Hemoglobin Carrier Based on Heulandite-Calcium Mesoporous Aluminosilicate Particles
- PMID: 35806461
- PMCID: PMC9267069
- DOI: 10.3390/ijms23137460
A Novel Artificial Hemoglobin Carrier Based on Heulandite-Calcium Mesoporous Aluminosilicate Particles
Abstract
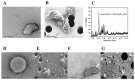
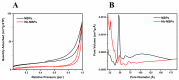
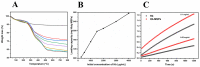
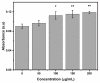
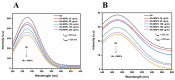
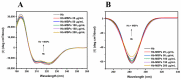
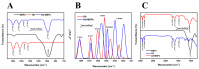
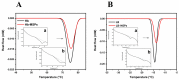
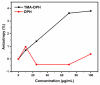
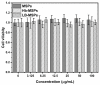
Tetraethyl-orthosilicate (TEOS)-based nanoparticles are most extensively used as a silica-based hemoglobin carrier system. However, TEOS-based nanoparticles induce adverse effects on the hemoglobin structure. Therefore, a heulandite-calcium-based carrier was investigated as a novel silica-based hemoglobin carrier system. The heulandite-calcium mesoporous aluminosilicate particles (MSPs) were fabricated by a patented tribo-mechanical activation process, according to the manufacturer, and its structure was assessed by X-ray diffraction analysis. Upon hemoglobin encapsulation, alternation in the secondary and tertiary structure was observed. The hemoglobin-particle interactions do not cause heme degradation or decreased activity. Once encapsulated inside the particle pores, the hemoglobin shows increased thermal stability, and higher loading capacity per gram of particles (by a factor of >1.4) when compared to TEOS-based nanoparticles. Futhermore, we introduced a PEGlyted lipid bilayer which significantly decreases the premature hemoglobin release and increases the colloidal stability. The newly developed hemoglobin carrier shows no cytotoxicity to human umbilical vein endothelial cells (HUVEC).
Keywords: encapsulation; hemoglobin carrier; mesoporous; silica particles; spectroscopy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Azimipour S., Ghaedi S., Mehrabi Z., Ghasemzadeh S.A., Heshmati M., Barikrow N., Attar F., Falahati M. Heme degradation and iron release of hemoglobin and oxidative stress of lymphocyte cells in the presence of silica nanoparticles. Int. J. Biol. Macromol. 2018;118:800–807. doi: 10.1016/j.ijbiomac.2018.06.128. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

