Electrochemical Detection and Analysis of Various Current Responses of a Single Ag Nanoparticle Collision in an Alkaline Electrolyte Solution
- PMID: 35806475
- PMCID: PMC9267213
- DOI: 10.3390/ijms23137472
Electrochemical Detection and Analysis of Various Current Responses of a Single Ag Nanoparticle Collision in an Alkaline Electrolyte Solution
Abstract
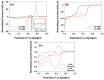
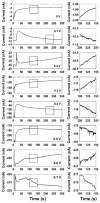
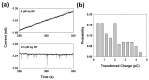
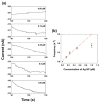
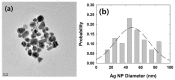
A single silver (Ag) nanoparticle (NP) collision was observed and analyzed in an alkaline solution using the electrocatalytic amplification (EA) method. Previously, the observation of a single Ag NP collision was only possible through limited methods based on a self-oxidation of Ag NPs or a blocking strategy. However, it is difficult to characterize the electrocatalytic activity of Ag NPs at a single NP level using a method based on the self-oxidation of Ag NPs. When using a blocking strategy, size analysis is difficult owing to the edge effect in the current signal. The fast oxidative dissolution of Ag NPs has been a problem for observing the staircase response of a single Ag NP collision signal using the EA method. In alkaline electrolyte conditions, Ag oxides are stable, and the oxidative dissolution of Ag NPs is sluggish. Therefore, in this study, the enhanced magnitude and frequency of the current response for single Ag NP collisions were obtained using the EA method in an alkaline electrolyte solution. The peak height and frequency of single Ag NP collisions were analyzed and compared with the theoretical estimation.
Keywords: alkaline solution; electrocatalytic amplification; electrochemistry; silver (Ag); single nanoparticle; single-molecule studies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Van Broekhuizen P., van Broekhuizen F., Cornelissen R., Reijnders L. Use of nanomaterials in the European construction industry and some occupational health aspects thereof. J. Nanoparticle Res. 2011;13:447–462. doi: 10.1007/s11051-010-0195-9. - DOI
-
- Bauer L.A., Birenbaum N.S., Meyer G.J. Biological applications of high aspect ratio nanoparticles. J. Mater. Chem. 2004;14:517–526. doi: 10.1039/b312655b. - DOI
-
- Chen J., Lim B., Lee E.P., Xia Y. Shape-controlled synthesis of platinum nanocrystals for catalytic and electrocatalytic applications. Nano Today. 2009;4:81–95. doi: 10.1016/j.nantod.2008.09.002. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

