Assessment of Angiogenesis and Cell Survivability of an Inkjet Bioprinted Biological Implant in an Animal Model
- PMID: 35806588
- PMCID: PMC9267737
- DOI: 10.3390/ma15134468
Assessment of Angiogenesis and Cell Survivability of an Inkjet Bioprinted Biological Implant in an Animal Model
Abstract
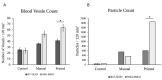
The rapidly growing field of tissue engineering hopes to soon address the shortage of transplantable tissues, allowing for precise control and fabrication that could be made for each specific patient. The protocols currently in place to print large-scale tissues have yet to address the main challenge of nutritional deficiencies in the central areas of the engineered tissue, causing necrosis deep within and rendering it ineffective. Bioprinted microvasculature has been proposed to encourage angiogenesis and facilitate the mobility of oxygen and nutrients throughout the engineered tissue. An implant made via an inkjet printing process containing human microvascular endothelial cells was placed in both B17-SCID and NSG-SGM3 animal models to determine the rate of angiogenesis and degree of cell survival. The implantable tissues were made using a combination of alginate and gelatin type B; all implants were printed via previously published procedures using a modified HP inkjet printer. Histopathological results show a dramatic increase in the average microvasculature formation for mice that received the printed constructs within the implant area when compared to the manual and control implants, indicating inkjet bioprinting technology can be effectively used for vascularization of engineered tissues.
Keywords: angiogenesis; bio-printing; inkjet printing; microvasculature; tissue engineering.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

