Correlation of Nabiximols Dose to Steady-State Concentrations of Cannabinoids in Urine Samples from Patients with Multiple Sclerosis
- PMID: 35807001
- PMCID: PMC9267351
- DOI: 10.3390/jcm11133717
Correlation of Nabiximols Dose to Steady-State Concentrations of Cannabinoids in Urine Samples from Patients with Multiple Sclerosis
Abstract
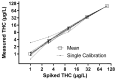
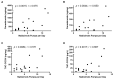
Therapeutic drug monitoring of Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD) is based on a complex procedure and is therefore not possible in most laboratories, especially in emergency cases. This work addresses the question of whether therapeutic drug monitoring of nabiximols can be performed using an immunological urine-based test system for cannabinoid abuse. Seventeen patients with multiple sclerosis were included in this study. Administered doses of nabiximols were correlated with immunologically determined urine concentrations of cannabinoids using the DRITM Cannabinoid (THC) Assay. Significant correlations with the administered nabiximols doses were found for creatinine-normalized urine concentrations of cannabinoids without (r = 0.675; p = 0.0015) and after (r = 0.650; p = 0.0044) hydrolysis, as well as for gas-chromatography-coupled mass spectrometry (GC/MS)-measured concentrations of the THC metabolite 11-nor-9-carboxy-Δ9-THC (THC-COOH) in urine samples (r = 0.571; p = 0.0084) by Pearson's correlation. In addition, doses were significantly correlated with plasma THC-COOH concentrations (r = 0.667; p = 0.0017) measured by GC/MS. Simple immunological cannabinoid measurements in urine samples could provide an estimate of nabiximols dosage, although the correlations obtained here were weak because of the small number of patients observed. Longitudinal monitoring of individual patients is expected to exhibit good results of therapeutic drug monitoring of nabiximols.
Keywords: DRITM Cannabinoid (THC) Assay; THC-COOH metabolite; drug monitoring; immunological test; multiple sclerosis; nabiximols; patients; urine assay.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Contin M., Mancinelli L., Perrone A., Sabattini L., Mohamed S., Scandellari C., Foschi M., Vacchiano V., Lugaresi A., Riva R. Tetrahydrocannabinol/Cannabidiol Oromucosal Spray in Patients with Multiple Sclerosis: A Pilot Study on the Plasma Concentration-Effect Relationship. Clin. Neuropharmacol. 2018;41:171–176. doi: 10.1097/WNF.0000000000000294. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

