Immunophenotypic Characteristics of Bone Marrow Microenvironment Cellular Composition at the Biochemical Progression of Multiple Myeloma
- PMID: 35807007
- PMCID: PMC9267252
- DOI: 10.3390/jcm11133722
Immunophenotypic Characteristics of Bone Marrow Microenvironment Cellular Composition at the Biochemical Progression of Multiple Myeloma
Abstract
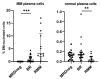
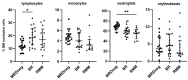
Multiple myeloma (MM) relapses are inevitable in the majority of patients, and in addition to genetic changes in the MM clone, the immune profile of the bone marrow (BM) plays a key role in this process. Biochemical progression or relapse (BR) precedes clinical relapse in a significant proportion of patients with MM. In the present study, we used flow cytometry to assess the cellular composition of the BM microenvironment in MM patients with confirmed BR. Fifteen distinct cells subsets in the BM were evaluated with the panel of antibodies used routinely for MRD monitoring in MM in 52 patients with MM (MRD-negative n = 20, BR n = 20, and clinically relapsed MM, RMM n = 12). The median percentage of MM cells detected in BR patients was 0.90% versus not detectable in MRD-negative patients and of 3.0% in RMM cohort. Compared to the MRD-negative group, BR status was associated with an increase in the percentage of lymphoid subpopulations, including memory B cells (p = 0.003), CD27+T cells (p = 0.002), and NK/NKT cells (p < 0.001). Moreover, a decrease in B-cell precursors (p < 0.001) and neutrophils (p = 0.006) was observed. There were no significant differences in the composition of the BM cell subpopulations between the BR and RMM groups. Our results indicate the involvement of B-, T-, and NK cells in the process of losing immune surveillance over the MM clone that leads to relapse. It can be speculated that similar studies of a larger cohort of BR patients can potentially identify a group of patients for which an early treatment intervention would be beneficial.
Keywords: biochemical relapse; immune profiling; microenvironment; multiple myeloma.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Flores-Montero J., Sanoja-Flores L., Paiva B., Puig N., García-Sánchez O., Böttcher S., van der Velden V.H.J., Pérez-Morán J.J., Vidriales M.B., García-Sanz R., et al. Next generation flow for highly sensitive and standardized detection of minimal residual disease in multiple myeloma. Leukemia. 2017;31:2094–2103. doi: 10.1038/leu.2017.29. - DOI - PMC - PubMed
-
- Munshi N.C., Avet-Loiseau H., Anderson K.C., Neri P., Paiva B., Samur M., Dimopoulos M., Kulakova M., Lam A., Hashim M., et al. Large meta-analysis establishes the role of MRD negativity in long-term survival outcomes in multiple myeloma patients. Blood Adv. 2020;4:5988–5999. doi: 10.1182/bloodadvances.2020002827. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

