The Ottawa Score Performs Poorly to Identify Cancer Patients at High Risk of Recurrent Venous Thromboembolism: Insights from the TROPIQUE Study and Updated Meta-Analysis
- PMID: 35807014
- PMCID: PMC9267563
- DOI: 10.3390/jcm11133729
The Ottawa Score Performs Poorly to Identify Cancer Patients at High Risk of Recurrent Venous Thromboembolism: Insights from the TROPIQUE Study and Updated Meta-Analysis
Abstract
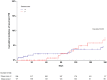
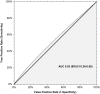
The Ottawa score (OS) for predicting the risk of recurrent venous thromboembolism (VTE) in cancer patients with VTE may help to guide anticoagulant treatment decisions that will optimize benefit-risk ratios. However, data on its reliability are conflicting. We applied the OS to all cancer patients with VTE enrolled in the prospective multicenter TROPIQUE study who received low-molecular-weight heparin over a 6-month period. Of 409 patients, 171 (41.8%) had a high-risk OS. The 6-month cumulative incidence of recurrent VTE was 7.8% (95%CI 4.2-14.8) in the high-risk OS group versus 4.8% (95%CI 2.6-8.9) in the low-risk OS group (SHR 1.47; 95%CI 0.24-8.55). The Area Under the Receiver Operating Characteristic curve (AUROC) of the OS in identifying patients who developed recurrent VTE was 0.53 (95%CI 0.38-0.65), and its accuracy was 57.9%. Among individual variables included in the OS, only prior VTE was significantly associated with the 6-month risk of recurrent VTE (SHR 4.39; 95% CI 1.13-17.04). When pooling data from all studies evaluating this score for predicting VTE recurrence in cancer patients (7 studies, 3413 patients), the OS estimated pooled AUROC was 0.59 (95%CI 0.56-0.62), and its accuracy was 55.7%. The present findings do not support the use of the OS to assess the risk of recurrent VTE in cancer patients.
Keywords: anticoagulants; cancer; recurrence; score; venous thromboembolism.
Conflict of interest statement
C.F. reports receiving honoraria for participating as a speaker at satellite symposia organized by Bayer, Bristol Myers Squibb, and LEO Pharma; other authors have nothing to disclose.
Figures

References
-
- Farge D., Debourdeau P., Beckers M., Baglin C., Bauersachs R.M., Brenner B., Brilhante D., Falanga A., Gerotzafias G.T., Haim N., et al. International Clinical Practice Guidelines for the Treatment and Prophylaxis of Venous Thromboembolism in Patients with Cancer. J. Thromb. Haemost. 2013;11:56–70. doi: 10.1111/jth.12070. - DOI - PubMed
-
- Farge D., Bounameaux H., Brenner B., Cajfinger F., Debourdeau P., Khorana A.A., Pabinger I., Solymoss S., Douketis J., Kakkar A. International Clinical Practice Guidelines Including Guidance for Direct Oral Anticoagulants in the Treatment and Prophylaxis of Venous Thromboembolism in Patients with Cancer. Lancet Oncol. 2016;17:e452–e466. doi: 10.1016/S1470-2045(16)30369-2. - DOI - PubMed
-
- Young A.M., Marshall A., Thirlwall J., Chapman O., Lokare A., Hill C., Hale D., Dunn J.A., Lyman G.H., Hutchinson C., et al. Comparison of an Oral Factor Xa Inhibitor with Low Molecular Weight Heparin in Patients with Cancer with Venous Thromboembolism: Results of a Randomized Trial (SELECT-D) J. Clin. Oncol. 2018;36:2017–2023. doi: 10.1200/JCO.2018.78.8034. - DOI - PubMed
-
- McBane R.D., Wysokinski W.E., Le-Rademacher J.G., Zemla T., Ashrani A., Tafur A., Perepu U., Anderson D., Gundabolu K., Kuzma C., et al. Apixaban and Dalteparin in Active Malignancy-Associated Venous Thromboembolism: The ADAM VTE Trial. J. Thromb. Haemost. 2020;18:411–421. doi: 10.1111/jth.14662. - DOI - PubMed
LinkOut - more resources
Full Text Sources

