Computational Prediction and Experimental Validation of the Unique Molecular Mode of Action of Scoulerine
- PMID: 35807231
- PMCID: PMC9268612
- DOI: 10.3390/molecules27133991
Computational Prediction and Experimental Validation of the Unique Molecular Mode of Action of Scoulerine
Abstract
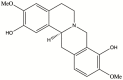
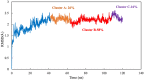
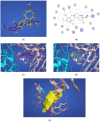
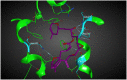
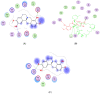
Scoulerine is a natural compound that is known to bind to tubulin and has anti-mitotic properties demonstrated in various cancer cells. Its molecular mode of action has not been precisely known. In this work, we perform computational prediction and experimental validation of the mode of action of scoulerine. Based on the existing data in the Protein Data Bank (PDB) and using homology modeling, we create human tubulin structures corresponding to both free tubulin dimers and tubulin in a microtubule. We then perform docking of the optimized structure of scoulerine and find the highest affinity binding sites located in both the free tubulin and in a microtubule. We conclude that binding in the vicinity of the colchicine binding site and near the laulimalide binding site are the most likely locations for scoulerine interacting with tubulin. Thermophoresis assays using scoulerine and tubulin in both free and polymerized form confirm these computational predictions. We conclude that scoulerine exhibits a unique property of a dual mode of action with both microtubule stabilization and tubulin polymerization inhibition, both of which have similar affinity values.
Keywords: cancer treatment; drug discovery; microtubule; molecular dynamic simulation; protein docking; scoulerine.
Conflict of interest statement
The authors declare no competing interests.
Figures








References
-
- Tian J., Mo J., Xu L., Zhang R., Qiao Y., Liu B., Jiang L., Ma S., Shi G. Scoulerine promotes cell viability reduction and apoptosis by activating ROS-dependent endoplasmic reticulum stress in colorectal cancer cells. Chem. Biol. Interact. 2020;327:109184. doi: 10.1016/j.cbi.2020.109184. - DOI - PubMed
-
- Kukula-Koch W.A., Widelski J. Alkaloids. In: Badal S., Delgoda R., editors. Pharmacognosy. Elsevier; Boston, MA, USA: 2017. pp. 163–198.
-
- Habartova K., Havelek R., Seifrtova M., Kralovec K., Cahlikova L., Chlebek J., Cermakova E., Mazankova N., Marikova J., Kunes J., et al. Scoulerine affects microtubule structure, inhibits proliferation, arrests cell cycle and thus culminates in the apoptotic death of cancer cells. Sci. Rep. 2018;8:4829. doi: 10.1038/s41598-018-22862-0. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

