Canady Cold Helios Plasma Reduces Soft Tissue Sarcoma Viability by Inhibiting Proliferation, Disrupting Cell Cycle, and Inducing Apoptosis: A Preliminary Report
- PMID: 35807413
- PMCID: PMC9268132
- DOI: 10.3390/molecules27134168
Canady Cold Helios Plasma Reduces Soft Tissue Sarcoma Viability by Inhibiting Proliferation, Disrupting Cell Cycle, and Inducing Apoptosis: A Preliminary Report
Abstract
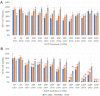
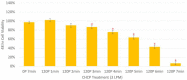
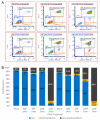
Soft tissue sarcomas (STS) are a rare and highly heterogeneous group of solid tumors, originating from various types of connective tissue. Complete removal of STS by surgery is challenging due to the anatomical location of the tumor, which results in tumor recurrence. Additionally, current polychemotherapeutic regimens are highly toxic with no rational survival benefit. Cold atmospheric plasma (CAP) is a novel technology that has demonstrated immense cancer therapeutic potential. Canady Cold Helios Plasma (CHCP) is a device that sprays CAP along the surgical margins to eradicate residual cancer cells after tumor resection. This preliminary study was conducted in vitro prior to in vivo testing in a humanitarian compassionate use case study and an FDA-approved phase 1 clinical trial (IDE G190165). In this study, the authors evaluate the efficacy of CHCP across multiple STS cell lines. CHCP treatment reduced the viability of four different STS cell lines (i.e., fibrosarcoma, synovial sarcoma, rhabdomyosarcoma, and liposarcoma) in a dose-dependent manner by inhibiting proliferation, disrupting cell cycle, and inducing apoptosis-like cell death.
Keywords: cancer treatment; cold atmospheric plasma; cold plasma device; fibrosarcoma; liposarcoma; rhabdomyosarcoma; soft tissue sarcoma; synovial sarcoma.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Ducimetière F., Lurkin A., Ranchère-Vince D., Decouvelaere A.-V., Peoc’h. M., Istier L., Chalabreysse P., Muller C., Alberti L., Bringuier P.-P., et al. Incidence of sarcoma histotypes and molecular subtypes in a prospective epidemiological study with central pathology review and molecular testing. PLoS ONE. 2011;6:e20294. doi: 10.1371/journal.pone.0020294. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

