Mixed-Valent Trinuclear CoIII-CoII-CoIII Complex with 1,3-Bis(5-chlorosalicylideneamino)-2-propanol
- PMID: 35807456
- PMCID: PMC9268718
- DOI: 10.3390/molecules27134211
Mixed-Valent Trinuclear CoIII-CoII-CoIII Complex with 1,3-Bis(5-chlorosalicylideneamino)-2-propanol
Abstract
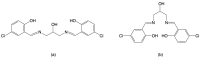
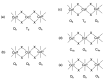
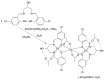
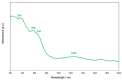
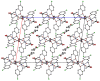
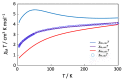
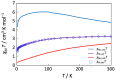
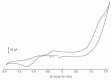
A mixed-valent trinuclear complex with 1,3-bis(5-chlorosalicylideneamino)-2-propanol (H3clsalpr) was synthesized, and the crystal structure was determined by the single-crystal X-ray diffraction method at 90 K. The molecule is a trinuclear CoIII-CoII-CoIII complex with octahedral geometries, having a tetradentate chelate of the Schiff-base ligand, bridging acetate, monodentate acetate coordination to each terminal Co3+ ion and four bridging phenoxido-oxygen of two Schiff-base ligands, and two bridging acetate-oxygen atoms for the central Co2+ ion. The electronic spectral feature is consistent with the mixed valent CoIII-CoII-CoIII. Variable-temperature magnetic susceptibility data could be analyzed by consideration of the axial distortion of the central Co2+ ion with the parameters Δ = -254 cm-1, λ = -58 cm-1, κ = 0.93, tip = 0.00436 cm3 mol-1, θ = -0.469 K, gz = 6.90, and gx = 2.64, in accordance with a large anisotropy. The cyclic voltammogram showed an irreversible reduction wave at approximately -1.2 V·vs. Fc/Fc+, assignable to the reduction of the terminal Co3+ ions.
Keywords: Schiff-base ligand; cobalt complex; mixed-valent complex; trinuclear complex.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Sinn E., Harris C.M. Schiff Base Metal Complexes as Ligands. Coord. Chem. Rev. 1969;4:391–422. doi: 10.1016/S0010-8545(00)80080-6. - DOI
-
- Calligaris M., Nardin G., Randaccio L. Structural aspects of metal complexes with some tetradentate Schiff bases. Coord. Chem. Rev. 1972;7:385–403. doi: 10.1016/S0010-8545(00)80018-1. - DOI
-
- Mederos A., Dominguez S., Hernandez-Molina R., Sanchiz J., Brito F. Coordinating ability of ligands derived from phenylenediamines. Coord. Chem. Rev. 1999;193–195:857–911. doi: 10.1016/S0010-8545(99)00050-8. - DOI
-
- Sakamoto M., Manseki K., Okawa H. d-f Heteronuclear complexes: Synthesis, structures and physicochemical aspects. Coord. Chem. Rev. 2001;219–221:379–414. doi: 10.1016/S0010-8545(01)00341-1. - DOI
-
- Vigato P.A., Tamburini S. The challenge of cyclic and acyclic schiff bases and related derivatives. Coord. Chem. Rev. 2004;248:1717–2128. doi: 10.1016/j.cct.2003.09.003. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

