Tirzepatide, a New Era of Dual-Targeted Treatment for Diabetes and Obesity: A Mini-Review
- PMID: 35807558
- PMCID: PMC9268041
- DOI: 10.3390/molecules27134315
Tirzepatide, a New Era of Dual-Targeted Treatment for Diabetes and Obesity: A Mini-Review
Erratum in
-
Correction: Chavda et al. Tirzepatide, a New Era of Dual-Targeted Treatment for Diabetes and Obesity: A Mini-Review. Molecules 2022, 27, 4315.Molecules. 2025 Mar 7;30(6):1190. doi: 10.3390/molecules30061190. Molecules. 2025. PMID: 40142172 Free PMC article.
Abstract
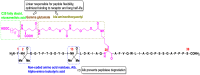
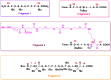
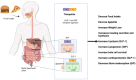
The prevalence of obesity and diabetes is an increasing global problem, especially in developed countries, and is referred to as the twin epidemics. As such, advanced treatment approaches are needed. Tirzepatide, known as a 'twincretin', is a 'first-in-class' and the only dual glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic peptide (GIP) receptor agonist, that can significantly reduce glycemic levels and improve insulin sensitivity, as well as reducing body weight by more than 20% and improving lipid metabolism. This novel anti-diabetic drug is a synthetic peptide analog of the human GIP hormone with a C20 fatty-diacid portion attached which, via acylation technology, can bind to albumin in order to provide a dose of the drug, by means of subcutaneous injection, once a week, which is appropriate to its a half-life of about five days. Tirzepatide, developed by Eli Lilly, was approved, under the brand name Mounjaro, by the United States Food and Drug Administration in May 2022. This started the 'twincretin' era of enormously important and appealing dual therapeutic options for diabetes and obesity, as well as advanced management of closely related cardiometabolic settings, which constitute the leading cause of morbidity, disability, and mortality worldwide. Herein, we present the key characteristics of tirzepatide in terms of synthesis, structure, and activity, bearing in mind its advantages and shortcomings. Furthermore, we briefly trace the evolution of this kind of medical agent and discuss the development of clinical studies.
Keywords: diabetes; incretins; obesity; short peptide; tirzepatide; twincretin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- World Health Organization Obesity and Overweight. 2021. [(accessed on 5 May 2022)]. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials