Evaluation of a High-Sensitivity Organ-Targeted PET Camera
- PMID: 35808181
- PMCID: PMC9269056
- DOI: 10.3390/s22134678
Evaluation of a High-Sensitivity Organ-Targeted PET Camera
Abstract
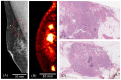
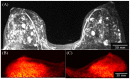
The aim of this study is to evaluate the performance of the Radialis organ-targeted positron emission tomography (PET) Camera with standardized tests and through assessment of clinical-imaging results. Sensitivity, count-rate performance, and spatial resolution were evaluated according to the National Electrical Manufacturers Association (NEMA) NU-4 standards, with necessary modifications to accommodate the planar detector design. The detectability of small objects was shown with micro hotspot phantom images. The clinical performance of the camera was also demonstrated through breast cancer images acquired with varying injected doses of 2-[fluorine-18]-fluoro-2-deoxy-D-glucose (18F-FDG) and qualitatively compared with sample digital full-field mammography, magnetic resonance imaging (MRI), and whole-body (WB) PET images. Micro hotspot phantom sources were visualized down to 1.35 mm-diameter rods. Spatial resolution was calculated to be 2.3 ± 0.1 mm for the in-plane resolution and 6.8 ± 0.1 mm for the cross-plane resolution using maximum likelihood expectation maximization (MLEM) reconstruction. The system peak noise equivalent count rate was 17.8 kcps at a 18F-FDG concentration of 10.5 kBq/mL. System scatter fraction was 24%. The overall efficiency at the peak noise equivalent count rate was 5400 cps/MBq. The maximum axial sensitivity achieved was 3.5%, with an average system sensitivity of 2.4%. Selected results from clinical trials demonstrate capability of imaging lesions at the chest wall and identifying false-negative X-ray findings and false-positive MRI findings, even at up to a 10-fold dose reduction in comparison with standard 18F-FDG doses (i.e., at 37 MBq or 1 mCi). The evaluation of the organ-targeted Radialis PET Camera indicates that it is a promising technology for high-image-quality, low-dose PET imaging. High-efficiency radiotracer detection also opens an opportunity to reduce administered doses of radiopharmaceuticals and, therefore, patient exposure to radiation.
Keywords: breast cancer; cancer detection; detectors; functional imaging; low-dose imaging; organ-targeted PET; precision medicine.
Conflict of interest statement
O.B., B.B., M.W., and A.R. have a financial interest in Radialis Medical. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures












References
-
- Wang X., Zhong X., Cheng L. Titanium-based nanomaterials for cancer theranostics. Coord. Chem. Rev. 2021;430:213662. doi: 10.1016/j.ccr.2020.213662. - DOI
-
- Miyake K.K., Nakamoto Y., Togashi K. Current Status of Dedicated Breast PET Imaging. Curr Radiol Rep. 2016;4:16. doi: 10.1007/s40134-016-0145-0. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

