Review on Modification of Glucomannan as an Excipient in Solid Dosage Forms
- PMID: 35808596
- PMCID: PMC9269564
- DOI: 10.3390/polym14132550
Review on Modification of Glucomannan as an Excipient in Solid Dosage Forms
Abstract
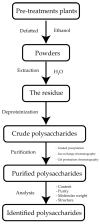
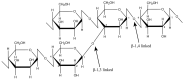
Glucomannan (GM)-a polysaccharide generally extracted from the tuber of Amorphophallus konjac-has great potential as a filler-binder in direct compression, disintegrant in tablets, or gelling agent due to its strong hydrophilicity and extremely high viscosity. However, it has poor water resistance and low mechanical strength when used as an excipient in solid form. Several physical and chemical modifications have been carried out to improve these drawbacks. Chemical modification affects the characteristics of GM based on the DS. Carboxymethylation improves GM functionality by modifying its solubility and viscosity, which in turn allows it to bind water more efficiently and thus improve its elongation and gel homogeneity. Meanwhile, physical modification enhances functionality through combination with other excipients to improve mechanical properties and modify swelling ability and drug release from the matrix. This review discusses extraction of GM and its modification to enhance its applicability as an excipient in solid form. Modified GM is a novel excipient applicable in the pharmaceutical industry for direct compression, as a tablet disintegrant, a film-forming agent, and for encapsulation of macromolecular compounds or drug carriers for controlled release.
Keywords: chemical modification; excipient; glucomannan; physical modification.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
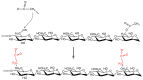
- Wang L.H., Huang G.Q., Xu T.C., Xiao J.X. Characterization of carboxymethylated konjac glucomannan for potential application in colon-targeted delivery. Food Hydrocoll. 2019;94:354–362. doi: 10.1016/j.foodhyd.2019.03.045. - DOI
-
- Xiao C., Weng L., Zhang L. Improvement of physical properties of crosslinked alginate and carboxymethyl konjac glucomannan blend films. J. Appl. Polym. Sci. 2002;84:2554–2560. doi: 10.1002/app.10582. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

