Role of Substrate Type in the Process of Polyelectrolyte Multilayer Formation
- PMID: 35808612
- PMCID: PMC9269168
- DOI: 10.3390/polym14132566
Role of Substrate Type in the Process of Polyelectrolyte Multilayer Formation
Abstract
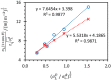
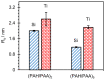
Polyelectrolyte multilayers are coatings formed by the alternate deposition of polycations and polyanions on a charged surface. In this study we examined how the type of substrate affects a multilayer prepared from poly(allylamine hydrochloride) and poly(acrylic acid). Silicon and titanium wafers were used as substrates. Their properties were systematically studied using ellipsometry, tensiometry, atomic force microscopy and streaming potential measurements. Multilayers were built up at pH = 7 with tetramethylammonium chloride as the background salt. The growth of films was monitored by ellipsometry, while the morphology and surface roughness were determined by atomic force microscopy. It was found that the thickness of multilayers containing 10 layers on silicon is 10 nm, whereas the thickness of the same film on titanium is three times higher. It was shown that multilayers formed on silicon display a grain-like structure, which was not the case for a film formed on titanium. Such morphological properties are also reflected in the surface roughness. Finally, it was shown that, in addition to the electrostatic interactions, the hydrophobicity of the substrate also plays an important role in the polyelectrolyte multilayer formation process and influences its thickness and properties.
Keywords: AFM; ellipsometry; polyelectrolyte multilayers; silicon; streaming potential; tensiometry; titanium.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Michaels A.S. Polyelectrolyte Complexes. Ind. Eng. Chem. 1965;57:32–40. doi: 10.1021/ie50670a007. - DOI
-
- Spruijt E., Leermakers F.A.M., Fokkink R., Schweins R., Van Well A.A., Cohen Stuart M.A., Van Der Gucht J. Structure and Dynamics of Polyelectrolyte Complex Coacervates Studied by Scattering of Neutrons, X-rays, and Light. Macromolecules. 2013;46:4596–4605. doi: 10.1021/ma400132s. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

