Supercritical Impregnation of Mango Leaf Extract into PLA 3D-Printed Devices and Evaluation of Their Biocompatibility with Endothelial Cell Cultures
- PMID: 35808751
- PMCID: PMC9269286
- DOI: 10.3390/polym14132706
Supercritical Impregnation of Mango Leaf Extract into PLA 3D-Printed Devices and Evaluation of Their Biocompatibility with Endothelial Cell Cultures
Abstract
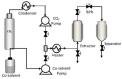
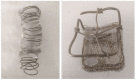
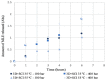
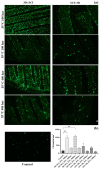
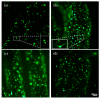
The addition of natural substances with pharmacoactive properties to polymeric biomedical devices would provide beneficial regarding the assimilation of these endoprostheses when implanted into a patient's body. The added drug would facilitate endothelization by regulating the inflammatory processes that such interventions entail, preventing contamination hazards and favoring the angiogenesis or formation of blood vessels in the tissue. The present work used mango leaf extract (MLE) obtained through pressurized ethanol for this purpose. Polylactic acid (PLA) in the form of filaments or 3D-printed disks was impregnated by means of supercritical technology with MLE for the culture essays. The release kinetics has been studied and the polymer matrices have been examined by scanning electron microscopy (SEM). The impregnated devices were subjected to in vitro culture of colony-forming endothelial cells. The influence of the different impregnation conditions used for the production of the MLE impregnated polymeric devices on the development of the cell culture was determined by fluorescence microscopy. The best results were obtained from the calcein cultures on 35 °C MLE impregnated into 3D-printed polymer disks.
Keywords: 3D printer; ECFCs; PLA; mango leaf; supercritical impregnation.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study, in the collection, analyses, or interpretation of the data, in the writing of the manuscript, or in the decision to publish the results.
Figures




References
-
- Nugroho W.T., Dong Y., Pramanik A. Dimensional accuracy and surface finish of 3D printed polyurethane (PU) dog-bone samples optimally manufactured by fused deposition modelling (FDM) Rapid Prototyp. J. 2022 doi: 10.1108/RPJ-12-2021-0328. in press . - DOI

