Engineered exosomes for studies in tumor immunology
- PMID: 35808839
- PMCID: PMC11044177
- DOI: 10.1111/imr.13107
Engineered exosomes for studies in tumor immunology
Abstract
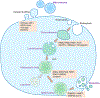
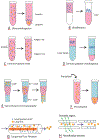
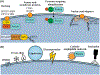
Exosomes are a type of extracellular vesicle (EV) with diameters of 30-150 nm secreted by most of the cells into the extracellular spaces and can alter the microenvironment through cell-to-cell interactions by fusion with the plasma membrane and subsequent endocytosis and release of the cargo. Because of their biocompatibility, low toxicity and immunogenicity, permeability (even through the blood-brain barrier (BBB)), stability in biological fluids, and ability to accumulate in the lesions with higher specificity, investigators have started making designer's exosomes or engineered exosomes to carry biologically active protein on the surface or inside the exosomes as well as using exosomes to carry drugs, micro RNA, and other products to the site of interest. In this review, we have discussed biogenesis, markers, and contents of various exosomes including exosomes of immune cells. We have also discussed the current methods of making engineered and designer's exosomes as well as the use of engineered exosomes targeting different immune cells in the tumors, stroke, as well as at peripheral blood. Genetic engineering and customizing exosomes create an unlimited opportunity to use in diagnosis and treatment. Very little use has been discovered, and we are far away to reach its limits.
Keywords: designer's exosomes; engineered exosomes; manipulation of biogenesis, exosomes, and immune cells; separation of exosomes; therapeutic exosomes.
© 2022 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Conflict of interest statement
CONFLICT OF INTEREST
None declared.
Figures


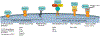




References
-
- van Niel G, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19:213. - PubMed
-
- Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654–659. - PubMed
-
- Denzer K, Kleijmeer MJ, Heijnen HF, Stoorvogel W, Geuze HJ. Exosome: from internal vesicle of the multivesicular body to intercellular signaling device. J Cell Sci. 2000;113(Pt 19):3365–3374. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

