Automation of radiation treatment planning for rectal cancer
- PMID: 35808871
- PMCID: PMC9512348
- DOI: 10.1002/acm2.13712
Automation of radiation treatment planning for rectal cancer
Abstract
Purpose: To develop an automated workflow for rectal cancer three-dimensional conformal radiotherapy (3DCRT) treatment planning that combines deep learning (DL) aperture predictions and forward-planning algorithms.
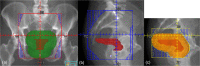
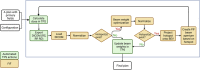
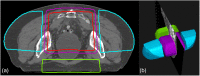
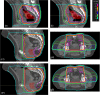
Methods: We designed an algorithm to automate the clinical workflow for 3DCRT planning with field aperture creations and field-in-field (FIF) planning. DL models (DeepLabV3+ architecture) were trained, validated, and tested on 555 patients to automatically generate aperture shapes for primary (posterior-anterior [PA] and opposed laterals) and boost fields. Network inputs were digitally reconstructed radiographs, gross tumor volume (GTV), and nodal GTV. A physician scored each aperture for 20 patients on a 5-point scale (>3 is acceptable). A planning algorithm was then developed to create a homogeneous dose using a combination of wedges and subfields. The algorithm iteratively identifies a hotspot volume, creates a subfield, calculates dose, and optimizes beam weight all without user intervention. The algorithm was tested on 20 patients using clinical apertures with varying wedge angles and definitions of hotspots, and the resulting plans were scored by a physician. The end-to-end workflow was tested and scored by a physician on another 39 patients.
Results: The predicted apertures had Dice scores of 0.95, 0.94, and 0.90 for PA, laterals, and boost fields, respectively. Overall, 100%, 95%, and 87.5% of the PA, laterals, and boost apertures were scored as clinically acceptable, respectively. At least one auto-plan was clinically acceptable for all patients. Wedged and non-wedged plans were clinically acceptable for 85% and 50% of patients, respectively. The hotspot dose percentage was reduced from 121% (σ = 14%) to 109% (σ = 5%) of prescription dose for all plans. The integrated end-to-end workflow of automatically generated apertures and optimized FIF planning gave clinically acceptable plans for 38/39 (97%) of patients.
Conclusion: We have successfully automated the clinical workflow for generating radiotherapy plans for rectal cancer for our institution.
Keywords: automation; deep learning; field-in-field; radiotherapy; rectal cancer.
© 2022 The Authors. Journal of Applied Clinical Medical Physics published by Wiley Periodicals, LLC on behalf of The American Association of Physicists in Medicine.
Conflict of interest statement
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Figures




References
-
- Kamran S, Mamon H, Wo J. Rectal and column cancer: radiation therapy planning. In: Hong T, Das P, eds. Radiation Therapy for Gastrointestinal Cancers. 1st ed. Springer International Publishing; 2017. Accessed October 15, 2020. 10.1007/978-3-319-43115-4 - DOI
-
- Herman J, Pawlik T. Colorectal cancer: radiation therapy for colorectal adenocarcinoma: external beam and intraoperative radiation therapy. In: Gearhart S, Ahuja N, eds. Early Diagnosis and Treatment of Cancer Series. Elsevier Health Sciences; 2010. https://books.google.com/books?id=dGbN5R‐r_WkC
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

