The emerging role of anti-PD-1 antibody-based regimens in the treatment of extranodal NK/T-cell lymphoma-associated hemophagocytic lymphohistiocytosis
- PMID: 35809114
- PMCID: PMC11797699
- DOI: 10.1007/s00432-022-04147-2
The emerging role of anti-PD-1 antibody-based regimens in the treatment of extranodal NK/T-cell lymphoma-associated hemophagocytic lymphohistiocytosis
Abstract
Purpose: Anti-PD-1 antibody (anti-PD-1 mAb) showed favorable outcomes in some patients with relapsed/refractory (r/r) extranodal NK/T-cell lymphoma (ENKTL). However, the role of anti-PD-1 antibody in NK/T-cell lymphoma-associated hemophagocytic lymphohistiocytosis (NK/T-LAHS) remains unclear. Here, we evaluated the efficacy and toxicity of anti-PD-1 antibody-based treatment in NK/T-LAHS patients.
Methods: The clinical data of 98 patients diagnosed with NK/T-LAHS at Sun Yat-sen University Cancer Center and the First Affiliated Hospital of Guangdong Pharmaceutical University from May 2014 to November 2021 were retrospectively analyzed. All patients received anti-HLH [HLH-2004 (etoposide, dexamethasone, cyclosporine A) or DEP-based (liposomal doxorubicin, etoposide, methylprednisolone)] regimen and sequential anti-ENKTL chemotherapy (ChT) combined with anti-PD-1 antibody or not.
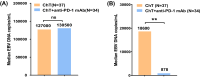
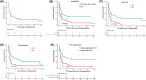
Results: The overall response rate (ORR) of the anti-PD-1 mAb plus ChT regimens was higher than that of the ChT regimens (73.3% vs. 45.5%, P = 0.041). The toxicity of the anti-PD-1 mAb plus ChT regimens was tolerable. Except for higher rate of neutropenia, no significant difference in adverse events (AEs) was observed between the two groups. When the optimal response to anti-ENKTL was achieved, the median EBV DNA levels in patients who received anti-PD-1 mAb plus ChT were significantly lower than patients who received ChT only (878 copies/mL vs. 18,600 copies/mL, P = 0.001). With a median follow-up of 26.6 months (range 0-65.9 months), the median overall survival (mOS) was 3.5 months (95% CI:2.3-4.7 months). Patients treated with anti-PD-1 mAb plus ChT experienced a longer mOS than those who received ChT only [5.2 months (95% CI: 2.5-7.8 months) vs. 1.5 months (95% CI: 0.5-2.6 months), P = 0.002]. Cox multivariate analysis found that anti-PD-1 mAb was an independent prognostic factor for all NK/T-LAHS patients.
Conclusion: In conclusion, anti-PD-1 mAb combined with ChT regimens seemed to be associated with prolonged survival in NK/T-LAHS patients and may represent a potentially promising treatment strategy for this population.
Keywords: Anti-PD-1 antibody; Epstein–Barr virus; Extranodal NK/T-cell lymphoma; Hemophagocytic lymphohistiocytosis.
© 2022. The Author(s), under exclusive licence to Springer-Verlag GmbH Germany, part of Springer Nature.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Using etoposide + dexamethasone-based regimens to treat nasal type extranodal natural killer/T-cell lymphoma-associated hemophagocytic lymphohistiocytosis.J Cancer Res Clin Oncol. 2021 Mar;147(3):863-869. doi: 10.1007/s00432-020-03376-7. Epub 2020 Oct 6. J Cancer Res Clin Oncol. 2021. PMID: 33025280 Free PMC article.
-
Ruxolitinib combined with doxorubicin, etoposide, and dexamethasone for the treatment of the lymphoma-associated hemophagocytic syndrome.J Cancer Res Clin Oncol. 2020 Nov;146(11):3063-3074. doi: 10.1007/s00432-020-03301-y. Epub 2020 Jul 2. J Cancer Res Clin Oncol. 2020. PMID: 32617699 Free PMC article.
-
Serum sCD25/ferritin ratio combined with MCP-1 is a valid predictor for identifying LAHS with HLH as the first manifestation.J Cancer Res Clin Oncol. 2023 Sep;149(11):8521-8533. doi: 10.1007/s00432-023-04781-4. Epub 2023 Apr 24. J Cancer Res Clin Oncol. 2023. PMID: 37093345 Free PMC article.
-
Nivolumab for adults with Hodgkin's lymphoma (a rapid review using the software RobotReviewer).Cochrane Database Syst Rev. 2018 Jul 12;7(7):CD012556. doi: 10.1002/14651858.CD012556.pub2. Cochrane Database Syst Rev. 2018. PMID: 30001476 Free PMC article.
-
Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: a network meta-analysis.Cochrane Database Syst Rev. 2020 Oct 19;10(10):CD012859. doi: 10.1002/14651858.CD012859.pub2. Cochrane Database Syst Rev. 2020. PMID: 33075160 Free PMC article.
Cited by
-
Primary prostatic Burkitt's lymphoma complicated with hemophagocytic lymphohistiocytosis: a case report and literature review.Front Oncol. 2025 Mar 17;15:1553415. doi: 10.3389/fonc.2025.1553415. eCollection 2025. Front Oncol. 2025. PMID: 40165892 Free PMC article.
-
Significance of whole-blood EBV DNA status in T/NK-cell lymphoma-associated hemophagocytic lymphohistiocytosis: a single-center retrospective analysis.Ther Adv Hematol. 2025 Feb 17;16:20406207251319604. doi: 10.1177/20406207251319604. eCollection 2025. Ther Adv Hematol. 2025. PMID: 39967833 Free PMC article.
-
A Nomogram Model Based on the Inflammation-Immunity-Nutrition Score (IINS) and Classic Clinical Indicators for Predicting Prognosis in Extranodal Natural Killer/T-Cell Lymphoma.J Inflamm Res. 2024 Apr 4;17:2089-2102. doi: 10.2147/JIR.S452521. eCollection 2024. J Inflamm Res. 2024. PMID: 38595337 Free PMC article.
-
Clinicopathological characteristics, prognostic factors, and outcomes of elderly patients with lymphoma-associated hemophagocytic lymphohistiocytosis: A multicenter analysis.Cancer Med. 2024 Aug;13(16):e70178. doi: 10.1002/cam4.70178. Cancer Med. 2024. PMID: 39219182 Free PMC article.
-
Clinical Efficacy of Programmed Cell Death Ligand 1 Antibody in Treatment of Extranodal Natural Killer/T-Cell Lymphoma With Hemophagocytic Lymphohistiocytosis.J Hematol. 2024 Apr;13(1-2):46-51. doi: 10.14740/jh1242. Epub 2024 Apr 9. J Hematol. 2024. PMID: 38644986 Free PMC article.
References
-
- Altorki N, McGraw T, Borczuk A, Saxena A, Port J, Stiles B, Lee B, Sanfilippo N, Scheff R, Pua B, Gruden J, Christos P, Spinelli C, Gakuria J, Uppal M, Binder B, Elemento O, Ballman K, Formenti S (2021) Neoadjuvant durvalumab with or without stereotactic body radiotherapy in patients with early-stage non-small-cell lung cancer: a single-centre, randomised phase 2 trial. Lancet Oncol 22(6):824–835. 10.1016/s1470-2045(21)00149-2 - PubMed
-
- Au WY, Weisenburger DD, Intragumtornchai T, Nakamura S, Kim WS, Sng I, Vose J, Armitage JO, Liang R (2009) Clinical differences between nasal and extranasal natural killer/T-cell lymphoma: a study of 136 cases from the International Peripheral T-Cell Lymphoma Project. Blood 113(17):3931–3937. 10.1182/blood-2008-10-185256 - PubMed
-
- Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R (2006) Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 439(7077):682–687. 10.1038/nature04444 - PubMed
-
- Bhatt NS, Oshrine B, An Talano J (2019) Hemophagocytic lymphohistiocytosis in adults. Leuk Lymphoma 60(1):19–28. 10.1080/10428194.2018.1482543 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

