The emerging role of anti-PD-1 antibody-based regimens in the treatment of extranodal NK/T-cell lymphoma-associated hemophagocytic lymphohistiocytosis
- PMID: 35809114
- PMCID: PMC11797699
- DOI: 10.1007/s00432-022-04147-2
The emerging role of anti-PD-1 antibody-based regimens in the treatment of extranodal NK/T-cell lymphoma-associated hemophagocytic lymphohistiocytosis
Abstract
Purpose: Anti-PD-1 antibody (anti-PD-1 mAb) showed favorable outcomes in some patients with relapsed/refractory (r/r) extranodal NK/T-cell lymphoma (ENKTL). However, the role of anti-PD-1 antibody in NK/T-cell lymphoma-associated hemophagocytic lymphohistiocytosis (NK/T-LAHS) remains unclear. Here, we evaluated the efficacy and toxicity of anti-PD-1 antibody-based treatment in NK/T-LAHS patients.
Methods: The clinical data of 98 patients diagnosed with NK/T-LAHS at Sun Yat-sen University Cancer Center and the First Affiliated Hospital of Guangdong Pharmaceutical University from May 2014 to November 2021 were retrospectively analyzed. All patients received anti-HLH [HLH-2004 (etoposide, dexamethasone, cyclosporine A) or DEP-based (liposomal doxorubicin, etoposide, methylprednisolone)] regimen and sequential anti-ENKTL chemotherapy (ChT) combined with anti-PD-1 antibody or not.
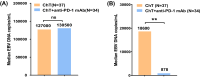
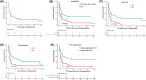
Results: The overall response rate (ORR) of the anti-PD-1 mAb plus ChT regimens was higher than that of the ChT regimens (73.3% vs. 45.5%, P = 0.041). The toxicity of the anti-PD-1 mAb plus ChT regimens was tolerable. Except for higher rate of neutropenia, no significant difference in adverse events (AEs) was observed between the two groups. When the optimal response to anti-ENKTL was achieved, the median EBV DNA levels in patients who received anti-PD-1 mAb plus ChT were significantly lower than patients who received ChT only (878 copies/mL vs. 18,600 copies/mL, P = 0.001). With a median follow-up of 26.6 months (range 0-65.9 months), the median overall survival (mOS) was 3.5 months (95% CI:2.3-4.7 months). Patients treated with anti-PD-1 mAb plus ChT experienced a longer mOS than those who received ChT only [5.2 months (95% CI: 2.5-7.8 months) vs. 1.5 months (95% CI: 0.5-2.6 months), P = 0.002]. Cox multivariate analysis found that anti-PD-1 mAb was an independent prognostic factor for all NK/T-LAHS patients.
Conclusion: In conclusion, anti-PD-1 mAb combined with ChT regimens seemed to be associated with prolonged survival in NK/T-LAHS patients and may represent a potentially promising treatment strategy for this population.
Keywords: Anti-PD-1 antibody; Epstein–Barr virus; Extranodal NK/T-cell lymphoma; Hemophagocytic lymphohistiocytosis.
© 2022. The Author(s), under exclusive licence to Springer-Verlag GmbH Germany, part of Springer Nature.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Altorki N, McGraw T, Borczuk A, Saxena A, Port J, Stiles B, Lee B, Sanfilippo N, Scheff R, Pua B, Gruden J, Christos P, Spinelli C, Gakuria J, Uppal M, Binder B, Elemento O, Ballman K, Formenti S (2021) Neoadjuvant durvalumab with or without stereotactic body radiotherapy in patients with early-stage non-small-cell lung cancer: a single-centre, randomised phase 2 trial. Lancet Oncol 22(6):824–835. 10.1016/s1470-2045(21)00149-2 - DOI - PubMed
-
- Au WY, Weisenburger DD, Intragumtornchai T, Nakamura S, Kim WS, Sng I, Vose J, Armitage JO, Liang R (2009) Clinical differences between nasal and extranasal natural killer/T-cell lymphoma: a study of 136 cases from the International Peripheral T-Cell Lymphoma Project. Blood 113(17):3931–3937. 10.1182/blood-2008-10-185256 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

