Teprotumumab Divergently Alters Fibrocyte Gene Expression: Implications for Thyroid-associated Ophthalmopathy
- PMID: 35809263
- PMCID: PMC9516078
- DOI: 10.1210/clinem/dgac415
Teprotumumab Divergently Alters Fibrocyte Gene Expression: Implications for Thyroid-associated Ophthalmopathy
Abstract
Context: Teprotumumab, an IGF-I receptor (IGF-IR) inhibitor, is effective in thyroid-associated ophthalmopathy (TAO). The drug can modulate induction by TSH of IL-6 and IL-8 in CD34+ fibrocytes and their putative derivatives, CD34+ orbital fibroblasts (CD34+ OF). Fibrocytes express multiple thyroid autoantigens and cytokines implicated in TAO, which are downregulated by Slit2. Inflammation and disordered hyaluronan (HA) accumulation occur in TAO. Whether teprotumumab alters these processes directly in fibrocytes/CD34+ OF remains uncertain.
Objective: Determine teprotumumab effects on expression/synthesis of several TAO-relevant molecules in fibrocytes and GD-OF.
Design/setting/participants: Patients with TAO and healthy donors were recruited from an academic endocrine and oculoplastic practice.
Main outcome measures: Real-time PCR, specific immunoassays.
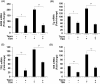
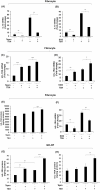
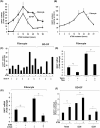
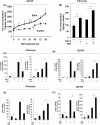
Results: Teprotumumab attenuates basal and TSH-inducible autoimmune regulator protein, thyroglobulin, sodium iodide symporter, thyroperoxidase, IL-10, and B-cell activating factor levels in fibrocytes. It downregulates IL-23p19 expression/induction while enhancing IL-12p35, intracellular and secreted IL-1 receptor antagonists, and Slit2. These effects are mirrored by linsitinib. HA production is marginally enhanced by teprotumumab, the consequence of enhanced HAS2 expression.
Conclusion: Teprotumumab affects specific gene expression in fibrocytes and GD-OF in a target-specific, nonmonolithic manner, whereas IGF-IR control of these cells appears complex. The current results suggest that the drug may act on cytokine expression and HA production systemically and locally, within the TAO orbit. These findings extend our insights into the mechanisms through which IGF-IR inhibition might elicit clinical responses in TAO, including a potential role of Slit2 in attenuating inflammation and tissue remodeling.
Keywords: Graves’ disease; cytokine; fibroblasts; hyaluronan; ophthalmopathy; orbit.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Endocrine Society. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures


References
-
- Kazim M, Goldberg RA, Smith TJ. Insights into the pathogenesis of thyroid-associated orbitopathy: evolving rationale for therapy. Arch Ophthalmol. 2002;120(3):380-386. - PubMed
-
- Smith TJ, Hegedus L. Graves’ disease. N Engl J Med. 2016;375(6):1552-1565. - PubMed
-
- Feliciello A, Porcellini A, Ciullo I, Bonavolonta G, Avvedimento EV, Fenzi G. Expression of thyrotropin-receptor mRNA in healthy and Graves’ disease retro-orbital tissue. Lancet. 1993;342(8867):337-338. - PubMed
-
- Heufelder AE, Dutton CM, Sarkar G, Donovan KA, Bahn RS. Detection of TSH receptor RNA in cultured fibroblasts from patients with Graves’ ophthalmopathy and pretibial dermopathy. Thyroid. 1993;3(4):297-300. - PubMed
-
- Smith TJ, Hoa N. Immunoglobulins from patients with Graves’ disease induce hyaluronan synthesis in their orbital fibroblasts through the self-antigen, insulin-like growth factor-I receptor. J Clin Endocrinol Metab. 2004;89(10):5076-5080. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

