Safety and immunogenicity of intramuscular, single-dose V590 (rVSV-SARS-CoV-2 Vaccine) in healthy adults: Results from a phase 1 randomised, double-blind, placebo-controlled, dose-ranging trial
- PMID: 35809371
- PMCID: PMC9259069
- DOI: 10.1016/j.ebiom.2022.104138
Safety and immunogenicity of intramuscular, single-dose V590 (rVSV-SARS-CoV-2 Vaccine) in healthy adults: Results from a phase 1 randomised, double-blind, placebo-controlled, dose-ranging trial
Abstract
Background: Vaccines against COVID-19 are needed to overcome challenges associated with mitigating the global pandemic. We report the safety and immunogenicity of V590, a live recombinant vesicular stomatitis virus-based COVID-19 vaccine candidate.
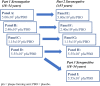
Methods: In this placebo-controlled, double-blind, three-part phase 1 study, healthy adults were randomised to receive a single intramuscular dose of vaccine or placebo. In Part 1, younger (18-54 years) and, in Part 2, older (≥55 years) adults seronegative for SARS-CoV-2 nucleocapsid received one of four V590 dose levels (5.00 × 105; 2.40 × 106; 1.15 × 107; or 5.55 × 107 plaque-forming units [pfu]) or placebo. In Part 3, a single V590 dose level (5.55 × 10⁷ pfu) or placebo was administered to younger SARS-CoV-2 seropositive adults. Primary endpoints included adverse events (AEs) and for Parts 1 and 2 anti-SARS-CoV-2 serum neutralising antibody responses measured by 50% plaque reduction neutralisation (PRNT50) assay at Day 28. Registration NCT04569786 [P001-02].
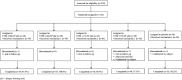
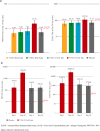
Findings: 232 participants were randomised and 219 completed the study. In seronegative participants, anti-SARS-CoV-2 spike-specific antibody responses to V590 were low and comparable to placebo across the lower dose levels. At the highest dose level (5.55 × 107 pfu), anti-SARS-CoV-2 spike-specific PRNT50 was 2.3-fold higher than placebo. The most frequently reported AEs were injection-site pain (38.4%), headache (15.1%) and fatigue (13.4%).
Interpretation: V590 was generally well-tolerated. However, Day 28 anti-SARS-Cov-2 spike-specific antibody responses in seronegative participants following a single intramuscular administration of V590 were not sufficient to warrant continued development.
Funding: The study was funded by Merck Sharp & Dohme LLC., a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
Keywords: COVID-19; SARS-CoV-2; V590; Vaccine; Vesicular stomatitis virus (VSV).
Copyright © 2022 Merck Sharp & Dohme Corp., a subsidiary Merck & Co., Inc., The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests JAR, QH, SD, TC, JC, JL, JS, CH, XZ, RW, EL, PA, DG and SAS are employees of Merck Sharpe & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA and may own stock or hold stock options in Merck & Co., Inc., Rahway, NJ, USA. JRS is an employee of Merck Sharpe & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA holding stocks alongside stocks in various pharmaceutical and biotechnology companies and service providers, has participated on various editorial boards and committees for Society for Industrial and Applied Mathematics and is a member and participant in committees for the International Society of Pharmacometrics. All other authors report no conflicts of interest.
Figures


References
-
- John Hopkins University & Medicine [Webpage]. COVID-19 dashboard by The Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). [Cited November 2021]. Available from: https://www.coronavirus.jhu.edu/map.html.
-
- Henao-Restrepo AM, Camacho A, Longini IM, et al. Efficacy and effectiveness of an rVSV-vectored vaccine in preventing Ebola virus disease: final results from the Guinea ring vaccination, open-label, cluster-randomised trial (Ebola Ça Suffit!) Lancet (London, England) 2017;389(10068):505–518. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

