Phase 2 study of anti-disialoganglioside antibody, dinutuximab, in combination with GM-CSF in patients with recurrent osteosarcoma: A report from the Children's Oncology Group
- PMID: 35809374
- PMCID: PMC9631806
- DOI: 10.1016/j.ejca.2022.05.035
Phase 2 study of anti-disialoganglioside antibody, dinutuximab, in combination with GM-CSF in patients with recurrent osteosarcoma: A report from the Children's Oncology Group
Abstract
Purpose: Novel effective therapies are urgently needed in recurrent osteosarcoma. GD2 is expressed in human osteosarcoma tumours and cell lines. This study evaluated the disease control rate (DCR) in patients with recurrent osteosarcoma treated with the anti-GD2 antibody dinutuximab plus cytokine therapy as compared to historical outcomes.
Methods: AOST1421 was a single-arm Phase 2 study for patients with recurrent pulmonary osteosarcoma in complete surgical remission. Patients received up to five cycles of dinutuximab (70 mg/m2/cycle) with granulocyte-macrophage colony-stimulating factor (GM-CSF). Two different dinutuximab infusion schedules were studied: 35 mg/m2/day over 20 h (2 days) and 17.5 mg/m2/day over 10 h (4 days). Primary end point was DCR, defined as a proportion of patients event free at 12 months from enrolment. The historical benchmark was 12-month DCR of 20% (95% CI 10-34%). Dinutuximab would be considered effective if ≥ 16/39 patients remained event free. Secondary objectives included toxicity evaluation and pharmacokinetics.
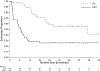
Results: Thirty-nine eligible patients were included in the outcome analysis. Dinutuximab did not demonstrate evidence of efficacy as 11/39 patients remained event free for a DCR of 28.2% (95% CI 15-44.9%). One of 136 administered therapy cycles met criteria for unacceptable toxicity when a patient experienced sudden death of unknown cause. Other ≥ Grade 3 toxicities included pain, diarrhoea, hypoxia, and hypotension. Pharmacokinetic parameters were similar in the two schedules.
Conclusions: The combination of dinutuximab with GM-CSF did not significantly improve DCR in recurrent osteosarcoma. Dinutuximab toxicity and pharmacokinetics in adolescent and young adult osteosarcoma patients were similar to younger patients. Other strategies for targeting GD2 in osteosarcoma are being developed.
Keywords: Dinutuximab; GD2; Recurrent osteosarcoma.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Conflict of interest statement The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: SGD has received consulting fees from Amgen, Bayer, and Jazz as well as travel expenses from Loxo, Roche, and Salarius. MDK has received consulting fees from Merck, Sharpe and Dhome. KAJ has received consulting fees from Bayer and Ipsen and honoraria from Takeda and Foundation Medicine. The remaining authors have no conflicts of interest.
Figures

References
-
- Bielack SS, Kempf-Bielack B, Branscheid D, Carrle D, Friedel G, Helmke K, et al. Second and subsequent recurrences of osteosarcoma: presentation, treatment, and outcomes of 249 consecutive cooperative osteosarcoma study group patients. J Clin Oncol 2009;27(4):557–65 doi 10.1200/JCO.2008.16.2305. - DOI - PubMed
-
- Heiner JP, Miraldi F, Kallick S, Makley J, Neely J, Smith-Mensah WH, et al. Localization of GD2-specific monoclonal antibody 3F8 in human osteosarcoma. Cancer Res 1987;47(20):5377–81. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

