Next generation GLP-1/GIP/glucagon triple agonists normalize body weight in obese mice
- PMID: 35809773
- PMCID: PMC9305623
- DOI: 10.1016/j.molmet.2022.101533
Next generation GLP-1/GIP/glucagon triple agonists normalize body weight in obese mice
Abstract
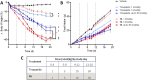
Objective: Pharmacological strategies that engage multiple mechanisms-of-action have demonstrated synergistic benefits for metabolic disease in preclinical models. One approach, concurrent activation of the glucagon-like peptide-1 (GLP-1), glucose-dependent insulinotropic peptide (GIP), and glucagon (Gcg) receptors (i.e. triagonism), combines the anorectic and insulinotropic activities of GLP-1 and GIP with the energy expenditure effect of glucagon. While the efficacy of triagonism in preclinical models is known, the relative contribution of GcgR activation remains unassessed. This work aims to addresses that central question.
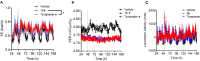
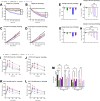
Methods: Herein, we detail the design of unimolecular peptide triagonists with an empirically optimized receptor potency ratio. These optimized peptide triagonists employ a protraction strategy permitting once-weekly human dosing. Additionally, we assess the effects of these peptides on weight-reduction, food intake, glucose control, and energy expenditure in an established DIO mouse model compared to clinically relevant GLP-1R agonists (e.g. semaglutide) and dual GLP-1R/GIPR agonists (e.g. tirzepatide).
Results: Optimized triagonists normalize body weight in DIO mice and enhance energy expenditure in a manner superior to that of GLP-1R mono-agonists and GLP-1R/GIPR co-agonists.
Conclusions: These pre-clinical data suggest unimolecular poly-pharmacology as an effective means to target multiple mechanisms contributing to obesity and further implicate GcgR activation as the differentiating factor between incretin receptor mono- or dual-agonists and triagonists.
Keywords: GIP; GLP-1; Glucagon; Obesity; Pharmacology; Triagonist.
Copyright © 2022 The Author(s). Published by Elsevier GmbH.. All rights reserved.
Figures


References
-
- Müller T.D., Clemmensen C., Finan B., DiMarchi R.D., Tschöp M.H. Anti-obesity therapy: from rainbow pills to polyagonists. Pharmacological Reviews. 2018;70(4):712–746. - PubMed
-
- Wilding J.P.H., Batterham R.L., Calanna S., Davies M., Van Gaal L.F., Lingvay I., et al. Once-weekly semaglutide in adults with overweight or obesity. New England Journal of Medicine. 2021;384(11):989–1002. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

