Circulating microRNAs as Reliable Tumor Biomarkers: Opportunities and Challenges Facing Clinical Application
- PMID: 35809898
- PMCID: PMC9827506
- DOI: 10.1124/jpet.121.000896
Circulating microRNAs as Reliable Tumor Biomarkers: Opportunities and Challenges Facing Clinical Application
Abstract
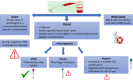
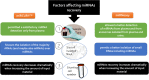
MicroRNAs (miRNAs) are involved in the development of human malignancies, and cells have the ability to secrete these molecules into extracellular compartments. Thus, cell-free miRNAs (circulating miRNAs) can potentially be used as biomarkers to evaluate pathophysiological changes. Although circulating miRNAs have been proposed as potential noninvasive tumor biomarkers for diagnosis, prognosis, and response to therapy, their routine application in the clinic is far from being achieved. This review focuses on the recent progress regarding the value of circulating miRNAs as noninvasive biomarkers, with specific consideration of their relevant clinical applications. In addition, we provide an in-depth analysis of the technical challenges that impact the assessment of circulating miRNAs. We also highlight the significance of integrating circulating miRNAs with the standard laboratory biomarkers to boost sensitivity and specificity. The current status of circulating miRNAs in clinical trials as tumor biomarkers is also covered. These insights and general guidelines will assist researchers in experimental practice to ensure quality standards and repeatability, thus improving future studies on circulating miRNAs. SIGNIFICANCE STATEMENT: Our review will boost the knowledge behind the inconsistencies and contradictory results observed among studies investigating circulating miRNAs. It will also provide a solid platform for better-planned strategies and standardized techniques to optimize the assessment of circulating cell-free miRNAs.
Copyright © 2022 by The American Society for Pharmacology and Experimental Therapeutics.
Figures
References
-
- Ali Ahmed E, Abd El-Basit SA, Mohamed MA, Swellam M (2020) Clinical role of MiRNA 29a and MiRNA 335 on breast cancer management: their relevance to MMP2 protein level. Arch Physiol Biochem 1–8. - PubMed
-
- Armand-Labit V, Pradines A (2017) Circulating cell-free microRNAs as clinical cancer biomarkers. Biomol Concepts 8:61–81. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical