Pexophagy is critical for fungal development, stress response, and virulence in Alternaria alternata
- PMID: 35810316
- PMCID: PMC9452759
- DOI: 10.1111/mpp.13247
Pexophagy is critical for fungal development, stress response, and virulence in Alternaria alternata
Abstract
Alternaria alternata can resist high levels of reactive oxygen species (ROS). The protective roles of autophagy or autophagy-mediated degradation of peroxisomes (termed pexophagy) against oxidative stress remain unclear. The present study, using transmission electron microscopy and fluorescence microscopy coupled with a GFP-AaAtg8 proteolysis assay and an mCherry tagging assay with peroxisomal targeting tripeptides, demonstrated that hydrogen peroxide (H2 O2 ) and nitrogen depletion induced autophagy and pexophagy. Experimental evidence showed that H2 O2 triggered autophagy and the translocation of peroxisomes into the vacuoles. Mutational inactivation of the AaAtg8 gene in A. alternata led to autophagy impairment, resulting in the accumulation of peroxisomes, increased ROS sensitivity, and decreased virulence. Compared to the wild type, ΔAaAtg8 failed to detoxify ROS effectively, leading to ROS accumulation. Deleting AaAtg8 down-regulated the expression of genes encoding an NADPH oxidase and a Yap1 transcription factor, both involved in ROS resistance. Deleting AaAtg8 affected the development of conidia and appressorium-like structures. Deleting AaAtg8 also compromised the integrity of the cell wall. Reintroduction of a functional copy of AaAtg8 in the mutant completely restored all defective phenotypes. Although ΔAaAtg8 produced wild-type toxin levels in axenic culture, the mutant induced a lower level of H2 O2 and smaller necrotic lesions on citrus leaves. In addition to H2 O2 , nitrogen starvation triggered peroxisome turnover. We concluded that ΔAaAtg8 failed to degrade peroxisomes effectively, leading to the accumulation of peroxisomes and the reduction of the stress response. Autophagy-mediated peroxisome turnover could increase cell adaptability and survival under oxidative stress and starvation conditions.
Keywords: Atg8; ROS detoxification; autophagy; peroxisome; pexophagy; stress tolerance; virulence.
© 2022 The Authors. Molecular Plant Pathology published by British Society for Plant Pathology and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no competing financial or nonfinancial interests.
Figures




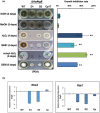
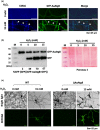


Similar articles
-
The Pex3-mediated peroxisome biogenesis plays a critical role in metabolic biosynthesis, stress response, and pathogenicity in Alternaria alternata.Microbiol Res. 2023 Jan;266:127236. doi: 10.1016/j.micres.2022.127236. Epub 2022 Oct 21. Microbiol Res. 2023. PMID: 36334316
-
PEX13 prevents pexophagy by regulating ubiquitinated PEX5 and peroxisomal ROS.Autophagy. 2023 Jun;19(6):1781-1802. doi: 10.1080/15548627.2022.2160566. Epub 2023 Jan 1. Autophagy. 2023. PMID: 36541703 Free PMC article.
-
A zinc finger suppressor involved in stress resistance, cell wall integrity, conidiogenesis, and autophagy in the necrotrophic fungal pathogen Alternaria alternata.Microbiol Res. 2022 Oct;263:127106. doi: 10.1016/j.micres.2022.127106. Epub 2022 Jun 30. Microbiol Res. 2022. PMID: 35839700
-
Pexophagy and Oxidative Stress: Focus on Peroxisomal Proteins and Reactive Oxygen Species (ROS) Signaling Pathways.Antioxidants (Basel). 2025 Jan 23;14(2):126. doi: 10.3390/antiox14020126. Antioxidants (Basel). 2025. PMID: 40002313 Free PMC article. Review.
-
Pexophagy in yeast and mammals: an update on mysteries.Histochem Cell Biol. 2018 Nov;150(5):473-488. doi: 10.1007/s00418-018-1724-3. Epub 2018 Sep 21. Histochem Cell Biol. 2018. PMID: 30238155 Review.
Cited by
-
The Emerging Role of Autophagy as a Target of Environmental Pollutants: An Update on Mechanisms.Toxics. 2023 Jan 30;11(2):135. doi: 10.3390/toxics11020135. Toxics. 2023. PMID: 36851010 Free PMC article. Review.
-
The Alternaria alternata StuA transcription factor interacting with the pH-responsive regulator PacC for the biosynthesis of host-selective toxin and virulence in citrus.Microbiol Spectr. 2023 Dec 12;11(6):e0233523. doi: 10.1128/spectrum.02335-23. Epub 2023 Oct 9. Microbiol Spectr. 2023. PMID: 37812002 Free PMC article.
-
Contribution of Autophagy to Cellular Iron Homeostasis and Stress Adaptation in Alternaria alternata.Int J Mol Sci. 2024 Jan 17;25(2):1123. doi: 10.3390/ijms25021123. Int J Mol Sci. 2024. PMID: 38256200 Free PMC article.
-
Transcriptome analysis of Aspergillus oryzae RIB40 under chemical stress reveals mechanisms of adaptation to fungistatic compounds of lignocellulosic side streams.Biotechnol Biofuels Bioprod. 2025 Aug 8;18(1):89. doi: 10.1186/s13068-025-02688-5. Biotechnol Biofuels Bioprod. 2025. PMID: 40781683 Free PMC article.
-
Molecular characterization of a new botybirnavirus that infects Alternaria sp. from tobacco.Arch Virol. 2024 Jun 18;169(7):149. doi: 10.1007/s00705-024-06072-w. Arch Virol. 2024. PMID: 38888750
References
-
- Biederbick, A. , Kern, H.F. & Elsasser, H.P. (1995) Monodansylcadaverine (MDC) is a specific in vivo marker for autophagic vacuoles. European Journal of Cell Biology, 66, 3–14. - PubMed
-
- Brocard, C. & Hartig, A. (2006) Peroxisome targeting signal 1: is it really a simple tripeptide? Biochimica et Biophysica Acta, 1763, 1565–1573. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources

