The effect of ivermectin on the viral load and culture viability in early treatment of nonhospitalized patients with mild COVID-19 - a double-blind, randomized placebo-controlled trial
- PMID: 35811080
- PMCID: PMC9262706
- DOI: 10.1016/j.ijid.2022.07.003
The effect of ivermectin on the viral load and culture viability in early treatment of nonhospitalized patients with mild COVID-19 - a double-blind, randomized placebo-controlled trial
Abstract
Objectives: Ivermectin, an antiparasitic agent, also has antiviral properties. In this study, we aimed to assess whether ivermectin has anti-SARS-CoV-2 activity.
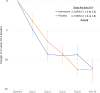
Methods: In this double-blinded trial, we compared patients receiving ivermectin for 3 days versus placebo in nonhospitalized adult patients with COVID-19. A reverse transcriptase-polymerase chain reaction from a nasopharyngeal swab was obtained at recruitment and every 2 days for at least 6 days. The primary endpoint was a reduction of viral load on the sixth day as reflected by cycle threshold level >30 (noninfectious level). The primary outcome was supported by the determination of viral-culture viability.
Results: Of 867 patients screened, 89 were ultimately evaluated per-protocol (47 ivermectin and 42 placeboes). On day 6, the odds ratio (OR) was 2.62 (95% confidence interval [CI]: 1.09-6.31) in the ivermectin arm, reaching the endpoint. In a multivariable logistic regression model, the odds of a negative test on day 6 were 2.28 times higher in the ivermectin group but reached significance only on day 8 (OR 3.70; 95% CI: 1.19-11.49, P = 0.02). Culture viability on days 2 to 6 was positive in 13.0% (3/23) of ivermectin samples versus 48.2% (14/29) in the placebo group (P = 0.008).
Conclusion: There were lower viral loads and less viable cultures in the ivermectin group, which shows its anti-SARS-CoV-2 activity. It could reduce transmission in these patients and encourage further studies with this drug.
Keywords: COVID-19; Infectivity duration; Ivermectin; SARS-CoV-2; Viral cultures.
Copyright © 2022. Published by Elsevier Ltd.
Figures


References
-
- Basso D, Aita A, Navaglia F, Franchin E, Fioretto P, Moz S, et al. SARS-CoV-2 RNA identification in nasopharyngeal swabs: issues in pre-analytics. Clin Chem Lab Med. 2020;58:1579–1586. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

