Relevant SARS-CoV-2 viremia is associated with COVID-19 severity: Prospective cohort study and validation cohort
- PMID: 35811284
- PMCID: PMC9349374
- DOI: 10.1002/jmv.27989
Relevant SARS-CoV-2 viremia is associated with COVID-19 severity: Prospective cohort study and validation cohort
Abstract
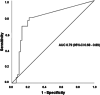
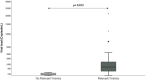
Early kinetics of SARS-CoV-2 viral load (VL) in plasma determined by quantitative reverse-transcription polymerase chain reaction (RT-PCR) was evaluated as a predictor of poor clinical outcome in a prospective study and assessed in a retrospective validation cohort. Prospective observational single-center study including consecutive adult patients hospitalized with COVID-19 between November 2020 and January 2021. Serial plasma samples were obtained until discharge. Quantitative RT-PCR was performed to assess SARS-CoV-2 VL. The main outcomes were in-hospital mortality, admission to the Intensive Care Unit (ICU), and their combination (Poor Outcome). Relevant viremia (RV), established in the prospective study, was assessed in a retrospective cohort including hospitalized COVID-19 patients from April 2021 to May 2022, in which plasma samples were collected according to clinical criteria. Prospective cohort: 57 patients were included. RV was defined as at least a twofold increase in VL within ≤2 days or a VL > 300 copies/ml, in the first week. Patients with RV (N = 14; 24.6%) were more likely to die than those without RV (35.7% vs. 0%), needed ICU admission (57% vs. 0%) or had Poor Outcome (71.4% vs. 0%), (p < 0.001 for the three variables). Retrospective cohort: 326 patients were included, 18.7% presented RV. Patients with RV compared with patients without RV had higher rates of ICU-admission (odds ratio [OR]: 5.6 [95% confidence interval [CI]: 2.1-15.1); p = 0.001), mortality (OR: 13.5 [95% CI: 6.3-28.7]; p < 0.0001) and Poor Outcome (OR: 11.2 [95% CI: 5.8-22]; p < 0.0001). Relevant SARS-CoV-2 viremia in the first week of hospitalization was associated with higher in-hospital mortality, ICU admission, and Poor Outcome. Findings observed in the prospective cohort were confirmed in a larger validation cohort.
Keywords: COVID-19; SARS-CoV-2; disease severity; poor outcome; viremia.
© 2022 The Authors. Journal of Medical Virology published by Wiley Periodicals LLC.
Conflict of interest statement
The authors of this manuscript have the following competing interests: Julio Ancochea reports grants and personal fees from GlaxoSmithKline and Boehringer Ingelheim; grants from Linde Healthcare; and grants, personal fees, and nonfinancial support from Roche and from Chiesi, outside the submitted work. Diego A. R. Serrano reports personal fees from MSD and Pfizer, outside the submitted work. Rafael de la Cámara reports personal fees from MSD, ASTELLAS, Clinigen, Janssen, Roche, and IQONE Health Care outside the submitted work. Rosario G. Vicuña reports grants, personal fees, and nonfinancial support from Abbvie, BMS, Lilly, Novartis, Sanofi, Sandoz, and MSD; personal fees from Biogen and Celltrion and from Mylan, outside the submitted work; personal fees and nonfinancial support from Pfizer; grants from Roche; and grants and personal fees from Janssen. Carmen S. Fernández reports personal fees from Bayer, BMS, Daichi Sankyo, MSD, and Pfizer, outside the submitted work. Cecilia M. Calleja reports competitive grants from ISCIII during the conduct of the study. Isidoro G. Álvaro reports grants from Instituto de Salud Carlos III, during the course of the study; Personal fees from Lilly and Sanofi; personal fees and nonfinancial support from BMS and Abbvie; research support, personal fees, and nonfinancial support from Roche Laboratories; and nonfinancial support from MSD, Pfizer, and Novartis, not related to the submitted work. The rest of the authors declare that they have no relevant conflicts of interests.
Figures




References
-
- COVID‐19 Map—Johns Hopkins Coronavirus Resource Center. Accessed February 14, 2022. https://coronavirus.jhu.edu/map.html
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

