Diagnostic Performance of Loop-Mediated Isothermal Amplification and Ultrasensitive Rapid Diagnostic Tests for Malaria Screening Among Pregnant Women in Kenya
- PMID: 35811308
- PMCID: PMC9585193
- DOI: 10.1093/infdis/jiac289
Diagnostic Performance of Loop-Mediated Isothermal Amplification and Ultrasensitive Rapid Diagnostic Tests for Malaria Screening Among Pregnant Women in Kenya
Abstract
Background: Screen-and-treat strategies with sensitive diagnostic tests may reduce malaria-associated adverse pregnancy outcomes. We conducted a diagnostic accuracy study to evaluate new point-of-care tests to screen pregnant women for malaria at their first antenatal visit in western Kenya.
Methods: Consecutively women were tested for Plasmodium infection by expert microscopy, conventional rapid diagnostic test (cRDT), ultra sensitive RDT (usRDT), and loop-mediated isothermal amplification (LAMP). Photoinduced electron-transfer polymerase chain reaction (PET-PCR) served as the reference standard. Diagnostic performance was calculated and modelled at low parasite densities.
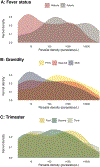
Results: Between May and September 2018, 172 of 482 screened participants (35.7%) were PET-PCR positive. Relative to PET-PCR, expert microscopy was least sensitive (40.1%; 95% confidence interval [CI], 32.7%-47.9%), followed by cRDT (49.4%; 95% CI, 41.7%-57.1), usRDT (54.7%; 95% CI, 46.9%-62.2%), and LAMP (68.6%; 95% CI, 61.1%-75.5%). Test sensitivities were comparable in febrile women (n = 90). Among afebrile women (n = 392), the geometric-mean parasite density was 29 parasites/µL and LAMP (sensitivity = 61.9%) and usRDT (43.2%) detected 1.74 (95% CI, 1.31-2.30) and 1.21 (95% CI, 88-2.21) more infections than cRDT (35.6%). Per our model, tests performed similarly at densities >200 parasites/µL. At 50 parasites/µL, the sensitivities were 45%, 56%, 62%, and 74% with expert microscopy, cRDT, usRDT, and LAMP, respectively.
Conclusions: This first-generation usRDT provided moderate improvement in detecting low-density infections in afebrile pregnant women compared to cRDTs.
Keywords: diagnostic sensitivity in malaria in pregnancy; loop-mediated isothermal amplicification for malaria; malaria in pregnancy; malaria screening at first antenatal care clinic visit; ultrasensitive rapid diagnostic tests for malaria.
Published by Oxford University Press on behalf of Infectious Diseases Society of America 2022.
Conflict of interest statement
Figures


References
-
- Rogerson SJ, Desai M, Mayor A, Sicuri E, Taylor SM, van Eijk AM. Burden, pathology, and costs of malaria in pregnancy: new developments for an old problem. Lancet Infect Dis 2018; 18:e107–e18. - PubMed
-
- Desai M, ter Kuile FO, Nosten F, et al. Epidemiology and burden of malaria in pregnancy. Lancet Infect Dis 2007; 7:93–104. - PubMed
-
- World Health Organization. A Strategic Framework for Malaria in Prevention and Control During Pregnancy in the African Region, 2004. AFR/MAL/04/01.
-
- Desai M, Hill J, Fernandes S, et al. Prevention of malaria in pregnancy. Lancet Infect Dis 2018; 18:e119–e32. - PubMed
Publication types
MeSH terms
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

