Bioinformatics roadmap for therapy selection in cancer genomics
- PMID: 35811332
- PMCID: PMC9627786
- DOI: 10.1002/1878-0261.13286
Bioinformatics roadmap for therapy selection in cancer genomics
Abstract
Tumour heterogeneity is one of the main characteristics of cancer and can be categorised into inter- or intratumour heterogeneity. This heterogeneity has been revealed as one of the key causes of treatment failure and relapse. Precision oncology is an emerging field that seeks to design tailored treatments for each cancer patient according to epidemiological, clinical and omics data. This discipline relies on bioinformatics tools designed to compute scores to prioritise available drugs, with the aim of helping clinicians in treatment selection. In this review, we describe the current approaches for therapy selection depending on which type of tumour heterogeneity is being targeted and the available next-generation sequencing data. We cover intertumour heterogeneity studies and individual treatment selection using genomics variants, expression data or multi-omics strategies. We also describe intratumour dissection through clonal inference and single-cell transcriptomics, in each case providing bioinformatics tools for tailored treatment selection. Finally, we discuss how these therapy selection workflows could be integrated into the clinical practice.
Keywords: bioinformatics; drug prioritisation; next-generation sequencing; precision oncology; treatment selection; tumour heterogeneity.
© 2022 The Authors. Molecular Oncology published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
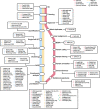
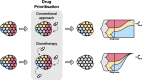
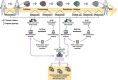
References
-
- US Food and Drug Administration . Table of pharmacogenomic biomarkers. 2022. [cited 2022 April 20]. Available from: http://www.fda.gov/drugs/science‐and‐research‐drugs/table‐pharmacogenomi...
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

