Computational study of ion permeation through claudin-4 paracellular channels
- PMID: 35811406
- PMCID: PMC9796105
- DOI: 10.1111/nyas.14856
Computational study of ion permeation through claudin-4 paracellular channels
Abstract
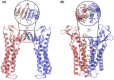
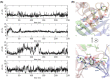
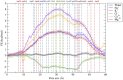
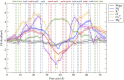
Claudins (Cldns) form a large family of protein homologs that are essential for the assembly of paracellular tight junctions (TJs), where they form channels or barriers with tissue-specific selectivity for permeants. In contrast to several family members whose physiological role has been identified, the function of claudin 4 (Cldn4) remains elusive, despite experimental evidence suggesting that it can form anion-selective TJ channels in the renal epithelium. Computational approaches have recently been employed to elucidate the molecular basis of Cldns' function, and hence could help in clarifying the role of Cldn4. In this work, we use structural modeling and all-atom molecular dynamics simulations to transfer two previously introduced structural models of Cldn-based paracellular complexes to Cldn4 to reproduce a paracellular anion channel. Free energy calculations for ionic transport through the pores allow us to establish the thermodynamic properties driving the ion-selectivity of the structures. While one model shows a cavity permeable to chloride and repulsive to cations, the other forms barrier to the passage of all the major physiological ions. Furthermore, our results confirm the charge selectivity role of the residue Lys65 in the first extracellular loop of the protein, rationalizing Cldn4 control of paracellular permeability.
Keywords: claudins; free energy calculations; ion-selectivity; molecular dynamics; paracellular space; tight junctions.
© 2022 The Authors. Annals of the New York Academy of Sciences published by Wiley Periodicals LLC on behalf of New York Academy of Sciences.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Anderson, J. M. (2001). Molecular structure of tight junctions and their role in epithelial transport. News in Physiological Sciences, 16, 126–130. - PubMed
-
- Gumbiner, B. (1987). Structure, biochemistry, and assembly of epithelial tight junctions. American Journal of Physiology‐Cell Physiology, 253, C749–C758. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

