Et tu, Neisseria? Conflicts of Interest Between Neisseria Species
- PMID: 35811666
- PMCID: PMC9263626
- DOI: 10.3389/fcimb.2022.913292
Et tu, Neisseria? Conflicts of Interest Between Neisseria Species
Abstract
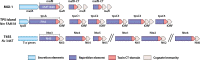
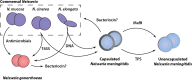
Neisseria meningitidis and Neisseria gonorrhoeae are two obligate human pathogens that have evolved to be uniquely adapted to their host. The meningococcus is frequently carried asymptomatically in the nasopharynx, while gonococcal infection of the urogenital tract usually elicits a marked local inflammatory response. Other members of the Neisseria genus are abundant in the upper airway where they could engage in co-operative or competitive interactions with both these pathogens. Here, we briefly outline the potential sites of contact between Neisseria spp. in the body, with emphasis on the upper airway, and describe the growing yet circumstantial evidence for antagonism from carriage studies and human volunteer challenge models with Neisseria lactamica. Recent laboratory studies have characterized antagonistic mechanisms that enable competition between Neisseria species. Several of these mechanisms, including Multiple Adhesin family (Mafs), Two Partner Secretion Systems, and Type VI secretion system, involve direct contact between bacteria; the genetic organisation of these systems, and the domain structure of their effector molecules have striking similarities. Additionally, DNA from one species of Neisseria can be toxic to another species, following uptake. More research is needed to define the full repertoire of antagonistic mechanisms in Neisseria spp., their distribution in strains, their range of activity, and contribution to survival in vivo. Understanding the targets of effectors could reveal how antagonistic relationships between close relatives shape subsequent interactions between pathogens and their hosts.
Keywords: Neisseria; antagonism; commensal; growth inhibition; pathogen.
Copyright © 2022 Baerentsen, Tang and Exley.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

