Efficacy of Bisphosphonate-Conjugated Sitafloxacin in a Murine Model of S. aureus Osteomyelitis: Evidence of "Target & Release" Kinetics and Killing of Bacteria Within Canaliculi
- PMID: 35811672
- PMCID: PMC9263620
- DOI: 10.3389/fcimb.2022.910970
Efficacy of Bisphosphonate-Conjugated Sitafloxacin in a Murine Model of S. aureus Osteomyelitis: Evidence of "Target & Release" Kinetics and Killing of Bacteria Within Canaliculi
Abstract
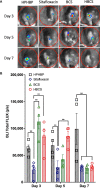
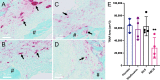
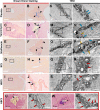
S. aureus infection of bone is difficult to eradicate due to its ability to colonize the osteocyte-lacuno-canalicular network (OLCN), rendering it resistant to standard-of-care (SOC) antibiotics. To overcome this, we proposed two bone-targeted bisphosphonate-conjugated antibiotics (BCA): bisphosphonate-conjugated sitafloxacin (BCS) and hydroxybisphosphonate-conjugate sitafloxacin (HBCS). Initial studies demonstrated that the BCA kills S. aureus in vitro. Here we demonstrate the in vivo efficacy of BCS and HBCS versus bisphosphonate, sitafloxacin, and vancomycin in mice with implant-associated osteomyelitis. Longitudinal bioluminescent imaging (BLI) confirmed the hypothesized "target and release"-type kinetics of BCS and HBCS. Micro-CT of the infected tibiae demonstrated that HBCS significantly inhibited peri-implant osteolysis versus placebo and free sitafloxacin (p < 0.05), which was not seen with the corresponding non-antibiotic-conjugated bisphosphonate control. TRAP-stained histology confirmed that HBCS significantly reduced peri-implant osteoclast numbers versus placebo and free sitafloxacin controls (p < 0.05). To confirm S. aureus killing, we compared the morphology of S. aureus autolysis within in vitro biofilm and infected tibiae via transmission electron microscopy (TEM). Live bacteria in vitro and in vivo presented as dense cocci ~1 μm in diameter. In vitro evidence of autolysis presented remnant cell walls of dead bacteria or "ghosts" and degenerating (non-dense) bacteria. These features of autolyzed bacteria were also present among the colonizing S. aureus within OLCN of infected tibiae from placebo-, vancomycin-, and sitafloxacin-treated mice, similar to placebo. However, most of the bacteria within OLCN of infected tibiae from BCA-treated mice were less dense and contained small vacuoles and holes >100 nm. Histomorphometry of the bacteria within the OLCN demonstrated that BCA significantly increased their diameter versus placebo and free antibiotic controls (p < 0.05). As these abnormal features are consistent with antibiotic-induced vacuolization, bacterial swelling, and necrotic phenotype, we interpret these findings to be the initial evidence of BCA-induced killing of S. aureus within the OLCN of infected bone. Collectively, these results support the bone targeting strategy of BCA to overcome the biodistribution limits of SOC antibiotics and warrant future studies to confirm the novel TEM phenotypes of bacteria within OLCN of S. aureus-infected bone of animals treated with BCS and HBCS.
Keywords: Staphylococcus aureus; antibiotic; bisphosphonate; osteomyelitis; transmission electron microscopy.
Copyright © 2022 Ren, Xue, Rainbolt, Bentley, Galloway, Liu, Cherian, Neighbors, Hofstee, Ebetino, Moriarty, Sun, Schwarz and Xie.
Conflict of interest statement
Authors PC, JN, FE and SS were employed by company BioVinc LLC. SS and FE hold equity in BioVinc LLC (Pasadena, CA) which partially sponsored this research. PC, SS, and FE are inventors on patents related to this work. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Evidence of bisphosphonate-conjugated sitafloxacin eradication of established methicillin-resistant S. aureus infection with osseointegration in murine models of implant-associated osteomyelitis.Bone Res. 2023 Oct 18;11(1):51. doi: 10.1038/s41413-023-00287-4. Bone Res. 2023. PMID: 37848449 Free PMC article.
-
Evidence of Bisphosphonate-Conjugated Sitafloxacin Eradication of Established Methicillin-Resistant S. aureus Infection with Osseointegration in Murine Models of Implant-Associated Osteomyelitis.Res Sq [Preprint]. 2023 May 11:rs.3.rs-2856287. doi: 10.21203/rs.3.rs-2856287/v1. Res Sq. 2023. Update in: Bone Res. 2023 Oct 18;11(1):51. doi: 10.1038/s41413-023-00287-4. PMID: 37214929 Free PMC article. Updated. Preprint.
-
Development of Bisphosphonate-Conjugated Antibiotics to Overcome Pharmacodynamic Limitations of Local Therapy: Initial Results with Carbamate Linked Sitafloxacin and Tedizolid.Antibiotics (Basel). 2021 Jun 17;10(6):732. doi: 10.3390/antibiotics10060732. Antibiotics (Basel). 2021. PMID: 34204351 Free PMC article.
-
Immune escape of Staphylococcus aureus mediated by osteocyte lacuna-canalicular network leads to persistent and uncured bone infection.Front Cell Infect Microbiol. 2025 Jun 2;15:1592086. doi: 10.3389/fcimb.2025.1592086. eCollection 2025. Front Cell Infect Microbiol. 2025. PMID: 40529302 Free PMC article. Review.
-
Mechanisms of Immune Evasion and Bone Tissue Colonization That Make Staphylococcus aureus the Primary Pathogen in Osteomyelitis.Curr Osteoporos Rep. 2019 Dec;17(6):395-404. doi: 10.1007/s11914-019-00548-4. Curr Osteoporos Rep. 2019. PMID: 31721069 Free PMC article. Review.
Cited by
-
Real-Time Impedance-Based Monitoring of the Growth and Inhibition of Osteomyelitis Biofilm Pathogen Staphylococcus aureus Treated with Novel Bisphosphonate-Fluoroquinolone Antimicrobial Conjugates.Int J Mol Sci. 2023 Jan 19;24(3):1985. doi: 10.3390/ijms24031985. Int J Mol Sci. 2023. PMID: 36768310 Free PMC article.
-
Advances in the targeted theragnostics of osteomyelitis caused by Staphylococcus aureus.Arch Microbiol. 2024 Jun 5;206(7):288. doi: 10.1007/s00203-024-04015-2. Arch Microbiol. 2024. PMID: 38834761 Review.
-
Evidence of bisphosphonate-conjugated sitafloxacin eradication of established methicillin-resistant S. aureus infection with osseointegration in murine models of implant-associated osteomyelitis.Bone Res. 2023 Oct 18;11(1):51. doi: 10.1038/s41413-023-00287-4. Bone Res. 2023. PMID: 37848449 Free PMC article.
-
Initial therapeutic evidence of a borosilicate bioactive glass (BSG) and Fe3O4 magnetic nanoparticle scaffold on implant-associated Staphylococcal aureus bone infection.Bioact Mater. 2024 Jun 8;40:148-167. doi: 10.1016/j.bioactmat.2024.05.040. eCollection 2024 Oct. Bioact Mater. 2024. PMID: 38962659 Free PMC article.
-
Bisphosphonate-Conjugated Sitafloxacin for Treatment of Staphylococcus aureus Infection Associated with Cortical Bone Screws: Case Series in Sheep Model.Pharmaceuticals (Basel). 2025 May 1;18(5):675. doi: 10.3390/ph18050675. Pharmaceuticals (Basel). 2025. PMID: 40430494 Free PMC article.
References
-
- Adjei-Sowah E., Peng Y., Weeks J., Jonason J. H., de Mesy Bentley K. L., Masters E., et al. . (2021). Development of Bisphosphonate-Conjugated Antibiotics to Overcome Pharmacodynamic Limitations of Local Therapy: Initial Results With Carbamate Linked Sitafloxacin and Tedizolid. Antibiotics (Basel) 10 (6), 732. doi: 10.3390/antibiotics10060732 - DOI - PMC - PubMed
-
- Chen Y., Hallab N. J., Liao Y. S., Narayan V., Schwarz E. M., Xie C. (2016). Antioxidant Impregnated Ultra-High Molecular Weight Polyethylene Wear Debris Particles Display Increased Bone Remodeling and a Superior Osteogenic:Osteolytic Profile vs. Conventional UHMWPE Particles in a Murine Calvaria Model. J. Orthop Res. 34 (5), 845–851. doi: 10.1002/jor.23080 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

