Commensal Urinary Lactobacilli Inhibit Major Uropathogens In Vitro With Heterogeneity at Species and Strain Level
- PMID: 35811675
- PMCID: PMC9260849
- DOI: 10.3389/fcimb.2022.870603
Commensal Urinary Lactobacilli Inhibit Major Uropathogens In Vitro With Heterogeneity at Species and Strain Level
Abstract
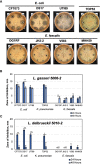
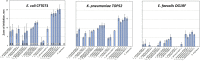
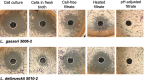
The human urinary microbiome is thought to affect the development and progression of urinary tract infections (UTI), particularly recurrent UTIs in aging populations of women. To understand the possible interactions of urinary pathogens with commensal bacteria inhabiting the aging bladder, we conducted an initial functional assessment of a representative set of urinary lactobacilli that dominate this niche in postmenopausal women. We created a repository of urinary bladder bacteria isolated via Enhanced Quantitative Urinary Culture (EQUC) from healthy postmenopausal women, as well as those with a culture-proven recurrent UTI (rUTI) diagnosis. This repository contains lactobacilli strains from eight different species. As many other lactobacilli are known to inhibit human pathogens, we hypothesized that some urinary lactobacilli will have similar abilities to inhibit the growth of typical uropathogens and thus, provide a link between the urinary microbiome and the predisposition to the rUTI. Therefore, we screened the urinary lactobacilli in our repository for their ability to inhibit model uropathogens in vitro. We observed that many urinary isolates strongly inhibit model strains of gram-negative Escherichia coli and Klebsiella pneumoniae but demonstrate less inhibition of gram-positive Enterococcus faecalis. The observed inhibition affected model strains of uropathogens as well as clinical and multidrug-resistant isolates of those species. Our preliminary analysis of inhibition modes suggests a combination of pH-dependent and cell-dependent inhibition. Overall, inhibition strongly varies among species and strains of urinary lactobacilli. While the strength of the inhibition is not predictive of health outcomes in this limited repository, there is a high level of species and strain diversity that warrants future detailed investigations.
Keywords: commensal lactobacilli; urinary lactobacilli; urinary microbiome; urinary tract infection; uropathogens.
Copyright © 2022 Johnson, Delaney, Ojha, Rudraraju, Hintze, Siddiqui and Sysoeva.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Atassi F., Pho Viet Ahn D. L., Lievin-Le Moal V. (2019). Diverse Expression of Antimicrobial Activities Against Bacterial Vaginosis and Urinary Tract Infection Pathogens by Cervicovaginal Microbiota Strains of Lactobacillus gasseri and Lactobacillus crispatus . Front. Microbiol. 10. doi: 10.3389/fmicb.2019.02900 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

