Glycation and a Spark of ALEs (Advanced Lipoxidation End Products) - Igniting RAGE/Diaphanous-1 and Cardiometabolic Disease
- PMID: 35811725
- PMCID: PMC9263181
- DOI: 10.3389/fcvm.2022.937071
Glycation and a Spark of ALEs (Advanced Lipoxidation End Products) - Igniting RAGE/Diaphanous-1 and Cardiometabolic Disease
Abstract
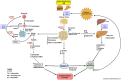
Obesity and non-alcoholic fatty liver disease (NAFLD) are on the rise world-wide; despite fervent advocacy for healthier diets and enhanced physical activity, these disorders persist unabated and, long-term, are major causes of morbidity and mortality. Numerous fundamental biochemical and molecular pathways participate in these events at incipient, mid- and advanced stages during atherogenesis and impaired regression of established atherosclerosis. It is proposed that upon the consumption of high fat/high sugar diets, the production of receptor for advanced glycation end products (RAGE) ligands, advanced glycation end products (AGEs) and advanced lipoxidation end products (ALEs), contribute to the development of foam cells, endothelial injury, vascular inflammation, and, ultimately, atherosclerosis and its consequences. RAGE/Diaphanous-1 (DIAPH1) increases macrophage foam cell formation; decreases cholesterol efflux and causes foam cells to produce and release damage associated molecular patterns (DAMPs) molecules, which are also ligands of RAGE. DAMPs stimulate upregulation of Interferon Regulatory Factor 7 (IRF7) in macrophages, which exacerbates vascular inflammation and further perturbs cholesterol metabolism. Obesity and NAFLD, characterized by the upregulation of AGEs, ALEs and DAMPs in the target tissues, contribute to insulin resistance, hyperglycemia and type two diabetes. Once in motion, a vicious cycle of RAGE ligand production and exacerbation of RAGE/DIAPH1 signaling ensues, which, if left unchecked, augments cardiometabolic disease and its consequences. This Review focuses on RAGE/DIAPH1 and its role in perturbation of metabolism and processes that converge to augur cardiovascular disease.
Keywords: RAGE axis; cardiometabolic disease; glycation; lipid metabolism; lipoxidation; non-alcoholic fatty liver disease; obesity.
Copyright © 2022 Arivazhagan, López-Díez, Shekhtman, Ramasamy and Schmidt.
Conflict of interest statement
RR, AS, and AMS have patents and patent applications through NYU Grossman School of Medicine that have been submitted/published that are related to some of the work reviewed in this manuscript. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Hudson BI, Kalea AZ, Del Mar Arriero M, Harja E, Boulanger E, D'Agati V, et al. . Interaction of the RAGE cytoplasmic domain with diaphanous-1 is required for ligand-stimulated cellular migration through activation of Rac1 and Cdc42. J Biol Chem. (2008) 283:34457–68. 10.1074/jbc.M801465200 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

