Reaction of Ta3 - Clusters with Molecular Nitrogen: A Mechanism Investigation
- PMID: 35811866
- PMCID: PMC9260930
- DOI: 10.1021/acsomega.2c02138
Reaction of Ta3 - Clusters with Molecular Nitrogen: A Mechanism Investigation
Abstract
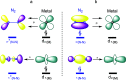
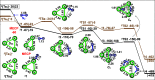
Because of the inertness of molecular nitrogen, its practicable activation under mild conditions is a fundamental challenge. Ta3 - is an exceptionally small cluster that reacts with N2 at room temperature, leading finally to Ta3N2 -; Ta3N2 - also could react with N2 at room temperature, leading finally to Ta3N4 -, a product of interest in its own right because of its potential as a nitrogen fixation medium. The mechanisms of the Ta3 -- and Ta3N2 --mediated activation of the N≡N triple bond have been investigated. Our extensive computations elucidate mechanisms for the ready reactions, leading to stepwise cleavage of the N≡N bond. Initial isomeric N2/Ta3 - complexes, N≡N elongation, undergo a N≡N split over generally low barriers in a highly exothermic process. The nitrogen-atom or molecular exchange reactions found in the Ta3N2 -/N2 system may be of paramount importance in both applied and fundamental studies.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Chertihin G. V.; Andrews L.; Bauschlicher C. W. Reactions of Laser-Ablated Scandium Atoms with Nitrogen: Matrix Infrared Spectra and DFT Calculations for Scandium Nitrides and the Fixation of Nitrogen by Two Scandium Atoms. J. Am. Chem. Soc. 1998, 120, 3205–3212. 10.1021/ja973701d. - DOI
LinkOut - more resources
Full Text Sources

