Effects of Acute Hypoxic Stress on Physiological and Hepatic Metabolic Responses of Triploid Rainbow Trout (Oncorhynchus mykiss)
- PMID: 35812328
- PMCID: PMC9263268
- DOI: 10.3389/fphys.2022.921709
Effects of Acute Hypoxic Stress on Physiological and Hepatic Metabolic Responses of Triploid Rainbow Trout (Oncorhynchus mykiss)
Abstract
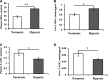
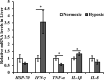
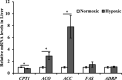
This experiment simulated the hypoxic environment caused by actual production operations in fish farming (i.e., catching, gathering, transferring, and weighting) to study the effects of acute hypoxic conditions on the physiological and metabolic responses of triploid rainbow trout (O. mykiss). Two groups of fish weighting 590 g were sampled in the normoxia group (dissolved oxygen above 7 mg/L) and hypoxia group (dissolved oxygen ranged from 2 to 5 mg/L for 10 min). The results showed that 1) regarding stress response, hypoxia increased plasma levels of cortisol, heat shock protein 70 (HSP-70), lysozyme, alanine aminotransferase (ALT), aspartate aminotransferase (AST) and creatine phosphokinase (CPK); induced the expression of hepatic genes encoding nuclear factor erythroid 2 related factor 2 (Nrf2), interferon γ (IFN-γ) and interleukin-1β (IL-1β). 2) Regarding metabolism response, hypoxia increased plasma levels of globulin (GLOB), glucose (GLU), triglyceride (TG) and lactate dehydrogenase (LDH); upregulated the hepatic gene expression of phosphoenolpyruvate carboxykinase, (PEPCK), pyruvate dehydrogenase kinase (PDK1), acetyl-CoA carboxylase (ACC) and acetyl-CoA oxidase (ACO); downregulated the hepatic gene expression of carnitine palmitoyl transferase 1 (CPT1); and unchanged the expression of hepatic genes in glycolysis and autophagy. 3) In response to hypoxia-inducible factors (HIFs), the hepatic HIF-2α gene was activated in the hypoxia group, but HIF-1α gene expression remained unchanged. Thus, during acute hypoxic stress, triploid rainbow trout were in a defensive state, with an enhanced immune response and altered antioxidant status. Additionally, the hepatic mitochondrial oxidation of glucose- and lipid-derived carbon in trout was suppressed, and hepatic gluconeogenesis and lipid synthesis were activated, which might be regulated by the HIF-2α pathway.
Keywords: HIF-2α; acute hypoxia; metabolism; physiology; triploid rainbow trout.
Copyright © 2022 Han, Meng, Tian, Li, Li, Gongbao, Fan and Ma.
Conflict of interest statement
Authors YL, CG, and WF were employed by Qinghai Minze Longyangxia Ecological Aquaculture Co., Ltd. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Characterization of a hypoxia-inducible factor (HIF-1alpha ) from rainbow trout. Accumulation of protein occurs at normal venous oxygen tension.J Biol Chem. 2001 Jun 8;276(23):19699-705. doi: 10.1074/jbc.M009057200. Epub 2001 Mar 9. J Biol Chem. 2001. PMID: 11278461
-
Temporal changes in stress and tissue-specific metabolic responses to municipal wastewater effluent exposure in rainbow trout.Comp Biochem Physiol C Toxicol Pharmacol. 2012 Aug;156(2):67-74. doi: 10.1016/j.cbpc.2012.04.002. Epub 2012 May 1. Comp Biochem Physiol C Toxicol Pharmacol. 2012. PMID: 22579662
-
Acute Stress and an Electrolyte- Imbalanced Diet, but Not Chronic Hypoxia, Increase Oxidative Stress and Hamper Innate Immune Status in a Rainbow Trout (Oncorhynchus mykiss) Isogenic Line.Front Physiol. 2019 Apr 24;10:453. doi: 10.3389/fphys.2019.00453. eCollection 2019. Front Physiol. 2019. PMID: 31068834 Free PMC article.
-
Metabolic dysregulation and emerging therapeutical targets for hepatocellular carcinoma.Acta Pharm Sin B. 2022 Feb;12(2):558-580. doi: 10.1016/j.apsb.2021.09.019. Epub 2021 Sep 25. Acta Pharm Sin B. 2022. PMID: 35256934 Free PMC article. Review.
-
Enzymes involved in l-lactate metabolism in humans.Mitochondrion. 2013 Nov;13(6):615-29. doi: 10.1016/j.mito.2013.08.011. Epub 2013 Sep 9. Mitochondrion. 2013. PMID: 24029012 Review.
Cited by
-
Rainbow trout welfare: comparing stunning methods in winter and summer.Fish Physiol Biochem. 2025 Jun 9;51(3):110. doi: 10.1007/s10695-025-01526-7. Fish Physiol Biochem. 2025. PMID: 40488916 Free PMC article.
-
Rainbow trout integrated response after recovery from short-term acute hypoxia.Front Physiol. 2022 Oct 21;13:1021927. doi: 10.3389/fphys.2022.1021927. eCollection 2022. Front Physiol. 2022. PMID: 36338491 Free PMC article.
-
Exploring the Effects of Acute Stress Exposure on Lumpfish Plasma and Liver Biomarkers.Animals (Basel). 2023 Nov 23;13(23):3623. doi: 10.3390/ani13233623. Animals (Basel). 2023. PMID: 38066974 Free PMC article.
-
Does Dietary Lipid Level Affect the Quality of Triploid Rainbow Trout and How Should It Be Assessed?Foods. 2022 Dec 21;12(1):15. doi: 10.3390/foods12010015. Foods. 2022. PMID: 36613231 Free PMC article.
-
Investigating the impact of camel whey protein hydrolysate on cyp1a1 and keap1/nrf2 expression in hypoxic stress-affected liver tissue of Oreochromis niloticus.J Comp Physiol B. 2025 Jun;195(3):323-338. doi: 10.1007/s00360-025-01623-2. Epub 2025 Jun 10. J Comp Physiol B. 2025. PMID: 40493086
References
-
- Akdemir F., Orhan C., Tuzcu M., Sahin N., Juturu V., Sahin K. (2016). The Efficacy of Dietary Curcumin on Growth Performance, Lipid Peroxidation and Hepatic Transcription Factors in Rainbow troutOncorhynchus Mykiss(Walbaum) Reared under Different Stocking Densities. Aquac. Res. 48, 4012–4021. 10.1111/are.13223 - DOI
-
- Barcellos L. J. G., Nicolaiewsky S., De Souza S. M. G., Lulhier F. (1999). The Effects of Stocking Density and Social Interaction on Acute Stress Response in Nile tilapia Oreochromis niloticus (L.) Fingerlings. Aquac. Res. 30, 887–892. 10.1046/j.1365-2109.1999.00419.x - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

