Exosome-mediated transduction of mechanical force regulates prostate cancer migration via microRNA
- PMID: 35812347
- PMCID: PMC9257336
- DOI: 10.1016/j.bbrep.2022.101299
Exosome-mediated transduction of mechanical force regulates prostate cancer migration via microRNA
Abstract
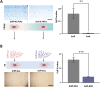
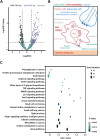
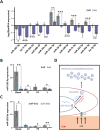
Physical cues in the extracellular microenvironment regulate cancer cell metastasis. Functional microRNA (miRNA) carried by cancer derived exosomes play a critical role in extracellular communication between cells and the extracellular microenvironment. However, little is known about the role of exosomes loaded miRNAs in the mechanical force transmission between cancer cells and extracellular microenvironment. Herein, our results suggest that stiff extracellular matrix (ECM) induced exosomes promote cancer cell migration. The ECM mechanical force regulated the exosome miRNA cargo of prostate cancer cells. Exosome miRNAs regulated by the ECM mechanical force modulated cancer cell metastasis by regulating cell motility, ECM remodeling and the interaction between cancer cells and nerves. Focal adhesion kinase mediated-ECM mechanical force regulated the intracellular miRNA expression, and F-actin mediate-ECM mechanical force regulated miRNA packaging into exosomes. The above results demonstrated that the exosome miRNA cargo promoted cancer metastasis by transmitting the ECM mechanical force. The ECM mechanical force may play multiple roles in maintaining the microenvironment of cancer metastasis through the exosome miRNA cargo.
Keywords: Cancer metastasis; Exosomes; Extracellular environment mechanical forces; MicroRNA; Prostate cancer.
© 2022 Naval Medical University. Published by Elsevier B.V.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
-
- Kechagia J.Z., Ivaska J., Roca-Cusachs P. Integrins as biomechanical sensors of the microenvironment. Nat. Rev. Mol. Cell Biol. 2019;20:457–473. - PubMed
LinkOut - more resources
Full Text Sources

