Strain Specific Variations in Acinetobacter baumannii Complement Sensitivity
- PMID: 35812377
- PMCID: PMC9258041
- DOI: 10.3389/fimmu.2022.853690
Strain Specific Variations in Acinetobacter baumannii Complement Sensitivity
Abstract
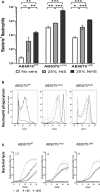
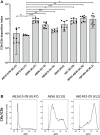
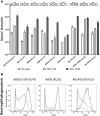
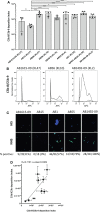
The complement system is required for innate immunity against Acinetobacter baumannii, an important cause of antibiotic resistant systemic infections. A. baumannii strains differ in their susceptibility to the membrane attack complex (MAC) formed from terminal complement pathway proteins, but the reasons for this variation remain poorly understood. We have characterized in detail the complement sensitivity phenotypes of nine A. baumannii clinical strains and some of the factors that might influence differences between strains. Using A. baumannii laboratory strains and flow cytometry assays, we first reconfirmed that both opsonization with the complement proteins C3b/iC3b and MAC formation were inhibited by the capsule. There were marked differences in C3b/iC3b and MAC binding between the nine clinical A. baumannii strains, but this variation was partially independent of capsule composition or size. Opsonization with C3b/iC3b improved neutrophil phagocytosis of most strains. Importantly, although C3b/iC3b binding and MAC formation on the bacterial surface correlated closely, MAC formation did not correlate with variations between A. baumannii strains in their levels of serum resistance. Genomic analysis identified only limited differences between strains in the distribution of genes required for serum resistance, but RNAseq data identified three complement-resistance genes that were differentially regulated between a MAC resistant and two MAC intermediate resistant strains when cultured in serum. These data demonstrate that clinical A. baumannii strains vary in their sensitivity to different aspects of the complement system, and that the serum resistance phenotype was influenced by factors in addition to the amount of MAC forming on the bacterial surface.
Keywords: Acinetobacter baumanniia; Gram negative bacteria; complement resistance/sensitivity; multi-drug resistance (MDR); virulence.
Copyright © 2022 Kamuyu, Ercoli, Ramos-Sevillano, Willcocks, Kewcharoenwong, Kiratisin, Taylor, Wren, Lertmemongkolchai, Stabler and Brown.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

