Alternative B Cell Differentiation During Infection and Inflammation
- PMID: 35812395
- PMCID: PMC9263372
- DOI: 10.3389/fimmu.2022.908034
Alternative B Cell Differentiation During Infection and Inflammation
Abstract
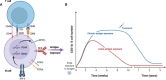
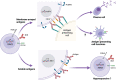
Long-term protective immunity to infectious disease depends on cell-mediated and humoral immune responses. Induction of a strong humoral response relies on efficient B cell activation and differentiation to long-lived plasma cells and memory B cells. For many viral or bacterial infections, a single encounter is sufficient to induce such responses. In malaria, the induction of long-term immunity can take years of pathogen exposure to develop, if it occurs at all. This repeated pathogen exposure and suboptimal immune response coincide with the expansion of a subset of B cells, often termed atypical memory B cells. This subset is present at low levels in healthy individuals as well but it is observed to expand in an inflammatory context during acute and chronic infection, autoimmune diseases or certain immunodeficiencies. Therefore, it has been proposed that this subset is exhausted, dysfunctional, or potentially autoreactive, but its actual role has remained elusive. Recent reports have provided new information regarding both heterogeneity and expansion of these cells, in addition to indications on their potential role during normal immune responses to infection or vaccination. These new insights encourage us to rethink how and why they are generated and better understand their role in our complex immune system. In this review, we will focus on recent advances in our understanding of these enigmatic cells and highlight the remaining gaps that need to be filled.
Keywords: CD11c; CD21; FcRL5; T-bet; atypical memory B cells; double-negative B cells; tissue-like B cells.
Copyright © 2022 Courey-Ghaouzi, Kleberg and Sundling.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

